|
# # # # News today of two biotech companies merging did not cause much of a ripple in the media, but the wider implications of the move are rather significant for Parkinson’s. Today it was announced that Brain Neurotherapy Bio (BNB) is going to merge with Asklepios Biopharmaceutical (aka AskBio). BNB are currently clinically testing a GDNF gene therapy approach for Parkinson’s, and AskBio is a subsidary of the large Pharmaceutical company Bayer. This is the same ‘Bayer’ that last year bought BlueRock Therapeutics – a biotech company focused on cell transplantation for Parkinson’s (Click here to read a previous SoPD post about that). In today’s post, we will discuss what BNB are doing and why this merger is particularly interesting. # # # # |
Source: BBRF
One of the themes this year on the SoPD website has been an effort to highlight (and encourage) more focus on alternative restorative therapies for Parkinson’s. There are a lot of different approaches exploring very different methods of slowing the progression of Parkinson’s, but most of the current clinical efforts investigating restorative therapies are oriented solely around cell transplantation.
What we really need are some novel strategies for replacing what is lost and encouraging re-growth from cells that remain.
Most of the SoPD posts exploring this idea during 2020 have been looking at very blue sky ideas (Click here, here, here and here to read some examples). But we have also been keeping an eye on biotech efforts in this domain, and today we received some interesting news which involved the merger of two biotech companies.
The merger occurred between Asklepios Biopharmaceutical (aka AskBio) and Brain Neurotherapy Bio.
ASKBio is a “gene therapy company dedicated to improving the lives of patients with rare diseases and other genetic disorders“. Gene therapy involves using DNA to treat medical conditions, rather than drugs. The DNA is usually delivered to the tissue requiring correction by carefully engineered viruses.
Brain Neurotherapy Bio is also a gene therapy biotech company that is currently clinically testing a GDNF gene therapy approach for Parkinson’s.
What is GDNF?
GDNF stands for glial cell line-derived neurotrophic factor.
Glial cells are the support cells in the brain. While neurons are considered to be the ‘work horses’ of neurological function – passing messages and storing memories – glial cells are in the background making sure that neurons are supported, protected and nurtured.
There are different types of glial cells, including astrocytes, oligodendrocytes and microglia. And each type has a specific function, for example microglia are the brain’s resident immune cells checking up on the health of the neurons while oligodendrocytes provide the neurons with a protective covering (called myelin sheath) which also helps to speed up the signalling of neurons.
 Different types of cells in the brain. Source: Dreamstime
Different types of cells in the brain. Source: Dreamstime
Astrocytes provide nutrients to neurons and make sure the environment surrounding the neurons is balanced and supportive. Glial cells are absolutely critical to the normal functioning of the brain.
The researchers that discovered GDNF found this protein in a cell culture of rat glial cells – hence the name: glial cell-line derived.
So that is the “glial cell-line” part of glial cell line-derived neurotrophic factor, now let’s focus on the latter part.
Neurotrophic factors (neurotrophic = Greek: neuron – nerve; trophikós – pertaining to food/to feed) are chemicals that nurture neurons and support growth. There are many types of neurotrophic factors, some having more beneficial effects on certain types of neurons and not other.
GDNF is one of these neurotrophic factors.
Of particular interest to us here at the SoPD is that GDNF is very neuroprotective for dopamine neurons (Click here for a very good OPEN ACCESS review of GDNF biology). Dopamine neurons are one group of cells in the brain that are badly affected by Parkinson’s. Thus, any protein that protects them and stops them from dying is of great interest to the Parkinson’s research community.
For readers not familiar with GDNF, it is important to state here that this neurotrophic factor has had a long and roller-coaster-like history with Parkinson’s. Before you read on, you might like to read a previous SoPD post that covers much of that history and recent clinical trial results (Click here to read that SoPD post).
|
# RECAP #1: Glial cell line-derived neurotrophic factor (or GDNF) is a neurotrophic factor – a naturally produced protein that nutures neurons, stimulating their growth and protecting them from harm. GDNF has demonstrated potent neuroprotective properties in preclinical studies involving dopamine neurons, which has led to the testing of this molecule in the context of Parkinson’s. # |
Ok, so Brain Neurotherapy Bio is a gene therapy biotech company working on GDNF. What is meant by “gene therapy”?
Gene therapy is an experimental treatment approach that involves treating medical conditions with DNA rather than drugs.
Gene therapy basically involves introducing a new piece of DNA or replacing a faulty piece of DNA within a population of cells. DNA, as you may remember from high school science class provides the instructions for making proteins in the a cell and these proteins are the bits that actually do stuff.
By introducing a new piece of DNA into a cell, the cell can start to produce a functioning protein that it may not normally produce. In some diseases, a cell may normally produce a particular protein, but because the genetic instructions in the DNA (a section of the DNA called a gene) for that protein have a small error (a genetic mutation), a non-functioning version of the protein is actually being produced. The introduction of the new correct (functioning) version of that piece of DNA (or gene) into a cell can start the production of a functional version of the protein.
 Gene therapy. Source: yourgenome
Gene therapy. Source: yourgenome
Alternatively, a gene can be introduced into a cell which would cause the cell to produce a protein that that cell usually does not produce. This sort of approach is being used in gene therapy for cancer, where ‘suicide genes’ are being introduced into cancer cells. These cause the cancer cell to die, by initiate an auto-destruct sequence resulting in cell death (a process called apoptosis). Another approach the cancer field is using is introducing a gene into cancer cells that cause a protein to be produced on the surface of the cancer cell that attracts the attention of the immune system. This ‘marker gene’ causes the immune system to attack the cancer cell, resulting in the death of the cancer cell.
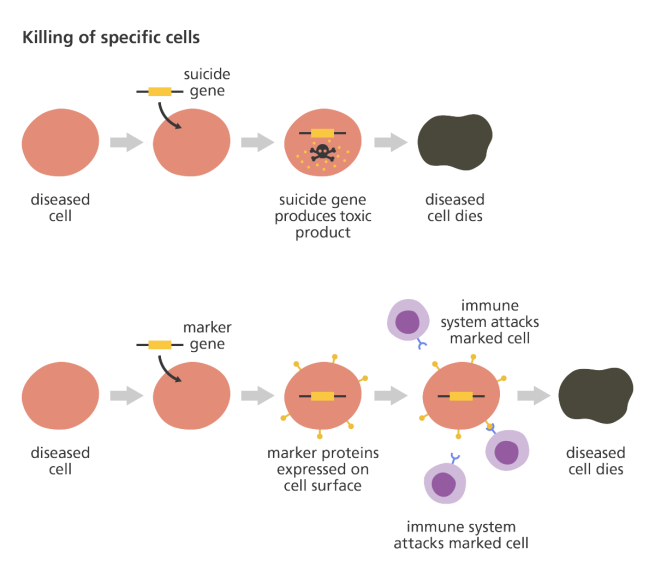 Source: yourgenome
Source: yourgenome
Taking this approach one step further, we can take sections of DNA that contain the genes involved with the production of a proteins that would be beneficial for the cell, such as GDNF. By then injecting a virus with the DNA for GDNF into the brain, we can produce GDNF in any infected cells (it’s slightly more complicated than that, but you get the basic idea).

Gene therapy for Parkinson’s disease. Source: Wiki.Epfl
I’m sorry, but did you say viruses?
Yes, if you remove the “disease-causing” DNA from inside any normal virus and replace it with something useful, that virus becomes a very useful biological delivery system. Far superior to anything we humans have devised thus far. Viruses are easy to produce and manipulate, and they can even be engineered to target specific cell types.
And these viruses have been engineered not to replicate. They deliver the proposed DNA and that is all.
I see. Has this gene therapy approach ever been tested in models of Parkinson’s?
Yes it has. Many times in fact.
Almost immediately after the discovery of GDNF was announced, researchers began trying to stick the DNA of GDNF into empty viruses with the goal of infecting cells in the brain and causing them to produce the protein. The first successful demonstration of this feat in a model of Parkinson’s was published in 1997:
 Title: Dopaminergic neurons protected from degeneration by GDNF gene therapy.
Title: Dopaminergic neurons protected from degeneration by GDNF gene therapy.
Authors: Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC.
Journal: Science. 1997 Feb 7;275(5301):838-41.
PMID: 9012352
In this study, the researchers inserted the DNA for GDNF into an adenovirus, and injected it into the part of the brain where the dopamine neurons reside (the substantia nigra). This treatment resulted in a 3 fold reduction in the loss of dopamine neurons 6 weeks after a neurotoxin (6-OHDA) was delivered (compared with no gene therapy or an empty virus control treatment).
And this initial GDNF-based gene therapy result was replicated by multiple independent labs very quickly (Click here and here for other examples).
But the type of virus used in gene therapy is important. Adenoviruses are known to cause immune responses in mammals. And this has caused researchers to shift to AAV viruses.
What are AAV viruses?
Adeno-associated viruses (or AAV) are a kind of virus that are popular with researchers because A.) they readily infect human and primate cells, and B.) they produce little (if any) immune response, and C.) they are non-pathogenic (they don’t cause any known diseases).
Given these characteristics, AAVs have been used in most of the gene therapy clinical trials thus far:
 AAV-based gene therapy clinical trials. Source: Wikipedia
AAV-based gene therapy clinical trials. Source: Wikipedia
They were originally discovered in the preparation of another type of virus, called an adenovirus (hence the name ‘Adeno-associated’). They were believed to simply be a contaminant of that preparation. Further research, however, revealed that AAVs belong to the Dependoparvo genus of viruses, which in turn belongs to the family Parvoviridae.
AAVs are single-stranded DNA viruses, and they are one of the smallest viruses (approximately 22 nm in diameter) with a non-enveloped capsid. The capsid is the shell surrounding the genetic material of the virus. Viruses are either enveloped or non-enveloped. “Enveloped” means that a second casing surrounds the capsid, providing further protection for the virus, while “non-enveloped” viruses have only the capsid.
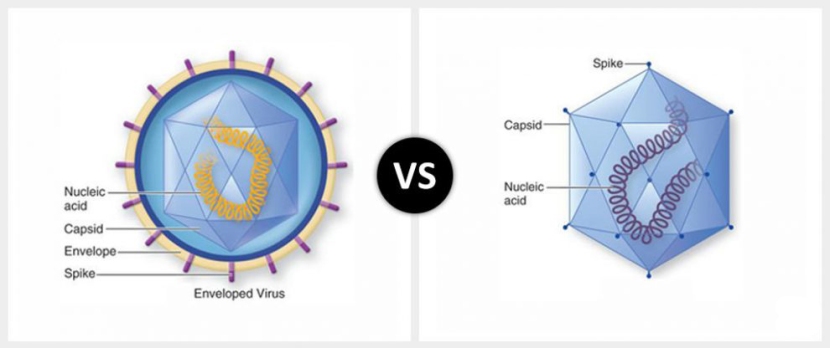
Enveloped (left) vs Non-enveloped (right) viruses. Source: Differencebtwn
Given the reduced amount of casing, non-enveloped viruses are generally more virulent (more infectious) than enveloped viruses (a good example of a non-enveloped virus is the influenza virus). Non-enveloped viruses do not survive outside of an organism for long though.

The AAV capsid. Source: Wikipedia
And AAV viruses have been found to be very effective at delivering GDNF DNA into models of Parkinson’s, for example:
 Title: Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats.
Title: Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats.
Authors: Mandel RJ, Spratt SK, Snyder RO, Leff SE.
Journal: Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):14083-8.
PMID: 9391156 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers injected of either an AAV GDNF virus or an AAV-control (empty) virus into the substantia nigra region of the rat brain 3 weeks prior to delivery of a neurotoxin (6-OHDA). When they anlysed the brains 4 weeks after the neurotoxin was administered, they found that the AAV GDNF virus protected the dopamine neurons significantly better than the AAV-control virus. The AAV GDNF virus treated animals had 94% of their dopamine neurons intact, compared to just 51% in the AAV-control virus treated animals.
All of these successful results of the GDNF-based gene therapy in models of Parkinson’s led researchers to test this approach in non-human primates (with the goal of ultimately testing it in humans). Despite the ethical issues surrounding the use of primates in research, health regulators still require new treatments to be tested in them before they will give the green light for clinical testing in humans.
The first demonstration of GDNF-based gene therapy in primates involved the delivery of a lentivirus containing GDNF DNA into a primate model of Parkinson’s. The results of that study were published in 2000:
 Title: Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease.
Title: Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease.
Authors: Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Déglon N, Aebischer P.
Journal: Science. 2000 Oct 27;290(5492):767-73.
PMID: 11052933
In this study, the researchers found that lentivirus-GDNF reversed the behavioural/motor deficits in a neurotoxin (MPTP)-based primate model of Parkinson’s and completely prevented dopamine neuron degeneration. And this lentivirus result has been replicated (Click here to read more about this).
But researchers have chosen to use AAV viruses in the clinical testing of GDNF-based gene therapy and this has also been tested in primates, with very positive results:
 Title: Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey
Title: Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey
Authors: Eslamboli A, Cummings RM, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Kirik D, Annett LE.
Journal: Exp Neurol. 2003 Nov;184(1):536-48.
PMID: 14637123
And all of these results collectively gave the research community confidence in taking GDNF gene therapy to clinical testing for Parkinson’s.
Hang on a second, these reports were published in 2000 and 2003. Why has the clinical trial taken so long?
Because at the time that these reports were being published, GDNF was being clinically tested in Parkinson’s using a direct administration of GDNF protein approach (tubes were inserted into the brains of participants and GDNF was periodically infused), which…. well, like I said above, the history of GDNF has been a roller coaster saga (Click here to read more about that).
|
# # RECAP #2: Gene therapy involves the use of DNA – rather than drugs – to treat a medical condition. The DNA is typically delivered to cells by carefully engineered viruses, which act as excellent biological delivery systems. Preclinical studies with GDNF gene therapy have provided interesting results and this is now being translated for evaluation in clinical trials. # # |
I see. So what has Brain Neurotherapy Bio done so far in clinical testing?
The research team behind Brain Neurotherapy Bio is being led by Prof Krystof Bankiewicz of Ohio State University.
 Prof Bankiewicz. Source: Swiatlekarza
Prof Bankiewicz. Source: Swiatlekarza
They have previously conducted one clinical trial evaluating GDNF gene therapy in Parkinson’s and the results of that study were published in late 2019:
 Title: Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson’s disease.
Title: Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson’s disease.
Authors: Heiss JD, Lungu C, Hammoud DA, Herscovitch P, Ehrlich DJ, Argersinger DP, Sinharay S, Scott G, Wu T, Federoff HJ, Zaghloul KA, Hallett M, Lonser RR, Bankiewicz KS.
Journal: Mov Disord. 2019 Jul;34(7):1073-1078.
PMID: 31145831 (This report is OPEN ACCESS if you would like to read it)
This was a Phase I, open-label pilot study, involved 13 people with advanced Parkinson’s. Each participant received a one-time injection of AAV2 virus containing GDNF-DNA injected into a region of the brain called the putamen – the injection was made on both sides of the head.
One quick question: what is the putamen?
As I mentioned above, dopamine neurons in the brain reside in an area called the substantia nigra, near the base of the brain, but they project their branches (or axons) to the several other areas, including the putamen, and this is where they release most of their dopamine.
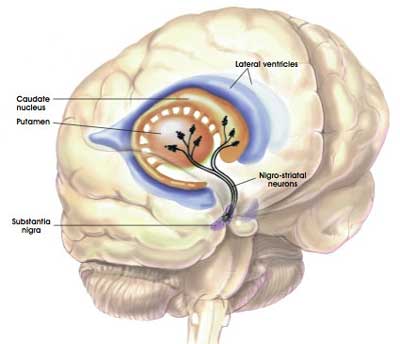 The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
Appreciating that the putamen is where many of the axons of the dopamine neurons can be found, the researchers hoped that by delivering GDNF to that region they would encourage the dopamine neurons to not only survive, but also to grow more branches. An example of regenerative medicine.
Previous research in models of Parkinson’s suggested that delivering GDNF to the substantia nigra protected the dopamine neurons, but not their branches. By delivering GDNF to the putamen, the researchers may be able to protect both the cell bodies and the branches.
Let’s continue:
The goal of the study was to investigate the safety, tolerability, and potential clinical effects of this gene therapy treatment. There were three different doses of GDNF AAV virus used in this study:
- a low dose (9 x 1010vg in 6 participants)
- a medium dose (3 x 1011vg in 6 participants)
- a high dose (9 x 1011vg in 1 participant))
Both pre-operatively and at 6-12 month intervals post-operatively, the participants in the study were clinically assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) to determine any improvement Parkinson’s symptoms. They were also given brain imaging (PET), which was conducted to measure any change in dopamine activity (Click here to read more about the details of this trial – though please note that some of the trial details have changed).
And what did the results suggest?
Importantly, the results indicate that the AAV2-GDNF treatment very safe and well tolerated. In addition, the UPDRS clinical assessment scores remained stable across the time frame of the study (important to remember here that this was an “open label” study – this means that everyone was aware that they were getting the experimental treatment (there was no placebo group) – thus a placebo response was possible).
Interestingly, in 12 of the 13 participants there was a 54% increase in dopamine activity at 18 months after AAV2-GDNF treatment (this effect ranged between 8-130% across the paritipants). This can be seen in the image below, where images in A represent where the gene therapy was delivered to in the brain (bright white blobs). Panel B was dopamine activity at baseline, and panel C was dopamine activity at 18 months post treatment (red indicates increases in activity).
 Source: PMC
Source: PMC
Again, it is important to remember that these initial results are based on an “open label” study, so it is difficult to determine if these findings are evidence of any actual disease modification. But the encouraging results did lead the researchers to set up a second clinical study, which was initiated in late 2019.
What do we know about that new study?
The new clinical trial is a Phase Ib study of AAV-GDNF and is taking place in the USA and Europe (specifically Warsaw, Poland). The new study differs slightly from the first Phase I study in that the researchers will be:
- Recruiting two groups of 6 participants for the study (one group of individuals with recently diagnosed Parkinson’s, and a second group of moderate-to-severe PD)
- Delivering the virus from the back of the skull, rather than the top of the skull (in order to cover more of the putamen)
- Increasing the vector volume (the volume is expected to triple in an effort to increase the coverage of the putamen to at least 50% – it was only 26% coverage in the first Phase I study)
By increasing the coverage of the putamen (that is, infecting more cells in the putamen with the AAV-GDNF virus treatment), the researchers are hoping to produce more GDNF. The thinking is that more GDNF will produce a better outcome.
This new study is also ‘open label’ with no placebo group or randomisation being involved. The study will be seeking to build more data to combine with the first Phase I study and determine if a larger double blinded Phase II study is justified in the future.
The primary endpoint (the predetermined measure of success) for the trial will be the number of adverse events (if any) that occur during the study. The secondary endpoints will include clinical evaluations of motor and non-motor features of Parkinson’s, as well as brain imaging of dopamine activity (DATScaN). The study is planned to finish in December 2022, but there will be a further follow up period – out to 2026 (Click here to read more about the details of the study).
|
# # # RECAP #3: A first clinical trial of GDNF gene therapy in Parkinson’s indicated that the treatment is safe and well tolerated in individuals with moderate to advanced PD. There were also encouraging results in the brain imaging data (suggesting increased dopamine activity). A second clinical trial is now being conducted in individuals with recently diagnosed Parkinson’s and those with moderate to advanced PD. # # # |
So why is it interesting that Brain Neurotherapy Bio merged with another biotech company?
It is interesting news because that other biotech company is Asklepios Biopharmaceutical (aka AskBio).
 AskBio is also a gene therapy biotech company, but – more importantly – it is a subsidary of the large pharmaceutical company Bayer.
AskBio is also a gene therapy biotech company, but – more importantly – it is a subsidary of the large pharmaceutical company Bayer.
 Does Bayer have a particular interest in Parkinson’s?
Does Bayer have a particular interest in Parkinson’s?
To date, no.
Bayer’s core business has primarily been focused on radiology, oncology and agricultural products (they currently only have one neurological product on the market which is Betaferon™ (Betaseron™) – an anti-inflammatory used for relapsing-remitting multiple sclerosis).
BUT, last year Bayer made a surprise move by buying BlueRock Therapeutics.
 BlueRock is a biotech company focused on cell transplantation for Parkinson’s (Click here to read a previous SoPD post about this). There were a lot of questions being asked in the grape vine about the strategy behind this move (“why is a radiology/agricultural pharma tying the knot with a stem cell company?”).
BlueRock is a biotech company focused on cell transplantation for Parkinson’s (Click here to read a previous SoPD post about this). There were a lot of questions being asked in the grape vine about the strategy behind this move (“why is a radiology/agricultural pharma tying the knot with a stem cell company?”).
Then in October this year, that Bayer had bought Asklepios Biopharmaceutical for US$2 billion (Click here to read more about this), and the speculation grew that Bayer was plotting a major change in future strategy (“a combination of gene therapy and cell transplantation?”).
We have previously discussed efforts being made by Aspen Neuroscience to genetically edit cells from patients before transplanting them into their brain as dopamine neurons (eg. correcting GBA variants in cells from individuals with GBA-associated PD – click here to read that SoPD post).
 Could Bayer be planning something similar?
Could Bayer be planning something similar?
The company have no product or marketed intellectual property in this area of medicine to be protecting, so their intentions can only be assumed to be well meaning. And they have written about it on their website (Click here to read an example), so they seem to be committed to this path.
 Source: Bayer
Source: Bayer
The move does not appear to be focused solely on Parkinson’s. The company has stated that it has a ‘vibrant’ cell and gene therapy pipeline with five advanced assets (including over 15 preclinical candidates). In addtition, Stefan Oelrich – a member of the Bayer AG board of management and president of the Pharmaceuticals Division has been quoted as saying “This is a defining moment for Bayer. Cell and gene therapies are leading innovation in healthcare, and it is our goal to be at the forefront of this revolution in science” (Source).
It will be interesting to see how this new merger plays out.
The hope is that Bayer will be able to allocate additional resources to these Parkinson’s programmes, speeding up their development. Both the cell transplantion and the gene therapy candidates are the closest products to market for both BlueRock and Brain Neurotherapy Bio, respectively. But both programmes are long term commitments (involving clinical trials with long follow up periods).
In addition, Werner Baumann (Bayer’s chief executive) lost a vote of no confidence earlier this year (Source), and there have been calls to break up the German conglomerate (Source), primarily on the back of a disasterous 2018 purchase of US agrochemicals group Monsanto for US$63bn, which has been plagued by legal battles over that company’s weedkiller “Roundup”. So while a long-term view must be taken on this stem cell/gene therapy strategic move, short-term business changes might dictate how things proceed.
Fingers crossed for some long-sighted future leadership.
So what does it all mean?
The quiet merger of two biotech companies has important implications for Parkinson’s research. The marriage of Asklepios Biopharmaceutical and Brain Neurotherapy Bio. barely caused a ripple in the news feed, but represents another piece in a major strategy change for the large pharmaceutical company Bayer. Importantly, they will be inheriting an exciting clinical trial programme that carries the hopes of many in the Parkinson’s community.
Long time readers will know that GDNF is a contenscious topic for a lot of folks in the Parkinson’s community – it represents hope to some and gets the blood boiling in others. As such, I was almost reluctant to write this post for fear of the consequences (raised expectations, etc). But this piece of news struck me as rather intriguing and full of potential, so I thought I’d share it here.
The news also comes as Herantis Pharma shared the results of their clinical trial of CDNF (another neurotrophic protein) in Parkinson’s (Click here to read more about this). The route of administration used in the CDNF trial (implantation of tubes into the brain) has been recently questioned and reviewed by the researchers at Herantis and they have chosen to take a less invasive approach in the next step of their clinical development of CDNF (Click here to read more about this).
This concern about the invasiveness of the implanted delivery of neurotrophic factors is shared by many researchers in the wider field – as discussed in a recent consensus report following the Bristol GDNF clinical trial results (Click here to read that report). The gene therapy approach does not require the permanent implantation of tubes in the brain, so it is more appealing from that standpoint – gene therapy requires a one time injection into the brain.
But there are cautionary notes associated with gene therapy, for example there is no off switch once the gene therapy is delivered, and it is also difficult to control the ‘volume’ of the new protein being produced (it lacks the tunability of deep brain stimulation). Such issues might not be a concern with the localised production of a neurotrophic factor (the individuals involved in the first GDNF gene therapy study are all still doing well), but it is important to keep such considerations in mind.
Enough said. Interesting developments, let’s see what happens next.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: Many of the companies mentioned in this website are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Brain Neurotherapy Bio and Bayer.


Excellent piece is always, Simon. These gene therapy open label trials present an interesting quandary to patients, especially in the context of some of these therapies like AAV2 AADC, which may have a higher probability of getting a regulatory approval in the near future, say two to three years.
If the GDNF works and further degeneration is slowed down or halted, it’s all well and good. If however for some reason it doesn’t work, it remains to be seen if patients would be eligible for AADC, which in my opinion has massive, massive quality of life implications. This question is especially compelling, considering that both GDNF and AADC are doing two of the same things. One, using the same viral serotype AAV2, which has the potential for the immune system to trigger a immune response the second time a gene therapy is administered, resulting in potentially poor gene expression. Second, The target tissue is the same, having the potential for tissue scarring and/or inflammation.
I’d like to know your thoughts
LikeLike