|
# # # # In September, a small biotech company called CuraSen announced that they had dosed the first participant in a clinical trial of their new experimental drug for Parkinson’s. This news did not garner a lot of attention, but was of great interest to us here at the SoPD because the drug – currently named CST-2032 – is the first of a novel class of drug to be tested in Parkinson’s. It also represents a shift in our approach to disease modification in neurodegenerative conditions (like Parkinson’s) as the focus moves away from solely being on the dopamine neurons. In today’s post, we will look at what CST-2032 is, what evidence exists that supports this drug going into clinical trial, and why it might represent a turning point in how we approach the treatment of Parkinson’s. # # # # |
The first thing you notice when you go to the CuraSen website are the words “Think, again“.
A curious introduction to a biotech, but it grabs the attention.
Next – and I don’t want to ruin things for anyone (Spoiler alert!) – the words fade away…
… only to be replaced by: “Rethinking neurodegeneration”
 At that point (if you are a curious creature) you start thinking: Ooh, this looks interesting.
At that point (if you are a curious creature) you start thinking: Ooh, this looks interesting.
And with a little bit of digging, you realise that it is interesting.
Very interesting.
Why is Curasen interesting?
Curasen is a California-based biotech taking a slightly different approach towards neurodegenerative conditions like Parkinson’s.
What are they doing?
Well, rather than looking at the brains of people with neurodegenerative conditions and focusing on the pathology – like alpha synuclein in the case of Parkinson’s – the researchers who founded Curasen went looking for the anatomical source of the problem.
And that search led them to the locus coeruleus.
The locus coer-what?!?
The locus coeruleus (which literally translates ‘blue place’) is a tiny structure in a region of the central nervous system called the brain stem. It is located at the top of the spinal cord and underneath the bulk of your brain – see image below:
 Location of the locus coeruleus. Source: Cell
Location of the locus coeruleus. Source: Cell
The neurons in the locus coeruleus project branches (called axons) to many different areas of your brain where they communicate with other neurons.
 Source: Neurology
Source: Neurology
The neurons in the locus coeruleus produce a neurotransmitter called norepinephrine.
Mmm, interesting. Two quick questions: What is a neurotransmitter? And what is norepinephrine?
Two quick answers:
A neurotransmitter is a signalling molecule – a way for neurons to communicate with each other. Neurotransmitters are released from neurons, and it binds to target neurons, initiating biological process within the receiving cell.
 A neurotransmitter being released by one neuron and binding to another. Source: Truelibido
A neurotransmitter being released by one neuron and binding to another. Source: Truelibido
One example of a neurotransmitter is the chemical dopamine, which is severely depleted in the brains of people with Parkinson’s.
 Dopamine. Source: Wikipedia
Dopamine. Source: Wikipedia
Another example of a neurotransmitter is norepinephrine, which (as you can see below) is very similar to dopamine:
 Norepinephrine. Source: Wikipedia
Norepinephrine. Source: Wikipedia
Norepinephrine (also called noradrenaline) is a neurotransmitter in the brain that is involved in cognition and motivation. Noradrenergic neurons also release norepinephrine into the blood system when the brain perceives that a stressful event has occurred and it acts as a stress hormone, increasing the heart rate and blood sugar levels to provide more energy for the body.
What happens to the locus coeruleus and norepinephrine levels in Parkinson’s?
The most characteristic pathological feature of the brain in an individual diagnosed with Parkinson’s is the loss of dopamine neurons. At the time of diagnosis, they will have lost approximately 50% of the dopamine producing neurons in a region of the brain called the substantia nigra. The disappearance of these dopamine neurons is associated with the appearance of the motor features of Parkinson’s.
The locus coeruleus sits below the substantia nigra in the brain stem and displays a similar level of cell loss in the Parkinsonian brain.
 Side on view of the human brainstem. Source: JNNP
Side on view of the human brainstem. Source: JNNP
The remaining noradrenergic neurons in the Parkinsonian brain often exhibit significant shrinkage, presenting smaller cell bodies (Click here to read more about this). In the image below, the panel on the left (C) shows noradrenergic neurons (the brown spots) in the locus coeruleus of an unaffected control brain, while the panel on the right (D) presents an image of noradrenergic neurons in the locus coeruleus from a Parkinsonian brain. Note the reduced number of brown spots (noradrenergic neurons) in panel D:
 Source: PMC
Source: PMC
The loss of the noradrenergic neurons in the locus coeruleus is associated with the development of non-motor features of Parkinson’s. To learn more about the noradrenergic system in Parkinson’s (Click here to read more about this).
One interesting detail here: The locus coeruleus is also badly affected in other neurodegenerative conditions, such as Alzheimer’s (Click here to read more about this).
|
# RECAP #1: The locus coeruleus is a small structure in the brain stem that contains neurons which produce the neurotransmitter norepinephrine. These neurons are involved in cognition and motivation Loss of locus coeruleus neurons is a common feature of both the Parkinsonian and Alzheimer’s brain. # |
Ok. So how is Curasen attempting to target the locus coeruleus?
The company’s approach is based on the research of Prof Mehrdad Shamloo from Stanford University.
 Prof Mehrdad Shamloo. Source: Stanford
Prof Mehrdad Shamloo. Source: Stanford
Prof Shamloo and colleagues have been very interested in molecules that modulate the activity of the noradrenergic neurons in the locus coeruleus.
Several years ago, they published this report:
 Title: beta1-adrenergic receptor activation enhances memory in Alzheimer’s disease model.
Title: beta1-adrenergic receptor activation enhances memory in Alzheimer’s disease model.
Authors: Coutellier L, Ardestani PM, Shamloo M.
Journal: Ann Clin Transl Neurol. 2014 May 1;1(5):348-360.
PMID: 24883337 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers demonstrated that modulation of the noradrenergic neurons could rescue memory deficits in mouse models of Alzheimer’s. And they have replicated these results in subsequent preclinical studies (Click here and here to read some examples).
How did they modulate the noradrenergic neurons???
Using a selective adrenoceptor agonist.
What is that?
An agonist is a drug that binds to and activates a particular receptor.
What is a receptor?
On the surface of a cell, there are lots of small molecules (called receptors) which act as switches for certain biological processes to be initiated. Receptors will wait for a protein to come along and bind to them, either activating them or alternatively blocking them (not allowing the biological process to be initiated).
The activators are called agonists, while the blockers are antagonists.
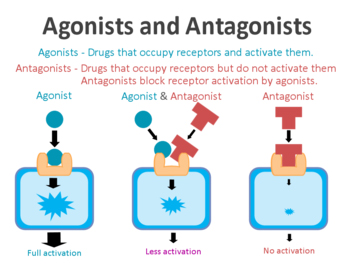 Agonist vs antagonist. Source: Psychonautwiki
Agonist vs antagonist. Source: Psychonautwiki
Ok. Got it. Agonists are drugs that activate receptors. What about the “adrenoceptor” part of “selective adrenoceptor agonist”?
Adrenoceptors is another name for adrenergic receptors. They are the receptors of neurotransmitters like norepinephrine (noradrenaline). There are different types of adrenoceptors, and drugs have been designed to selectively activate specific members of the adrenoceptor family.
And Curasen is a biotech company that is developing agonists for adrenergic receptors for neurodegenerative conditions like Parkinson’s.
Do we know if adrenoceptor agonists work in Parkinson’s?
This is a really interesting question. In 2017, this research report was published:
 Title: β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease
Title: β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease
Authors: Mittal S, Bjørnevik K, Im DS, Flierl A, Dong X, Locascio JJ, Abo KM, Long E, Jin M, Xu B, Xiang YK, Rochet JC, Engeland A, Rizzu P, Heutink P, Bartels T, Selkoe DJ, Caldarone BJ, Glicksman MA, Khurana V, Schüle B, Park DS, Riise T, Scherzer CR.
Journal: Science. 2017 Sep 1;357(6354):891-898.
PMID: 28860381 (This report is OPEN ACCESS if you would like to read it)
The researchers who conducted this study began by conducting a massive screening experiment. They wanted to identify drugs that could reduce the production of a protein called alpha synuclein (regular readers will be aware that that protein is intimately associated with Parkinson’s). So the investigators grew cells that stably produce the human version of alpha synuclein and they screened 1126 compounds (including drugs approved by the U.S. Food and Drug Administration (or FDA) as well as wide range of natural products, vitamins, health supplements, and alkaloids.
From this screen they identified 35 compounds that lowered alpha synuclein levels by more than 35%. Amongst these 35 compounds was the selective Beta2-Adrenoreceptor agonist metaproterenol (Note the ‘adrenoreceptor’ and ‘agonist’ part of that sentence).
Because metaproterenol is not brain penetrant – meaning that it can not pass through the protective blood-brain-barrier membrane surrounding the brain – the investigators added six related drugs, including the two selective Beta2-Adrenoreceptor agonists, clenbuterol and salbutamol (which are both brain penetrant).
Hang on a second, what are Beta2-adrenoreceptor agonist?
As I mentioned above, there are different kinds of adrenoreceptors. Beta2-adrenoreceptor agonist are drugs that bind to and activate the Beta2-adrenergic receptor. These receptors sit in the wall of cells (the membrane), with part of themselves exposed to the world outside the cells (the extracellular space) and another region of themselves exposed to the interior of the cell (the intracellular space).
 The Beta2-adrenergic receptor. Source: Wikipedia
The Beta2-adrenergic receptor. Source: Wikipedia
Beta2-adrenergic receptor interacts with norepinephrine, which is the neurotransmitter were discussing above that is produced by the neurons in the locus coeruleus. Norepinephrine will come floating along, bind to the Beta2-adrenergic receptor, and activate it.
Beta2-adrenoreceptor agonists are very similar to norepinephrine.
What medical conditions are treated with Beta2-adrenoreceptor agonists?
They are primarily used to treat pulmonary disorders – that is, conditions of the lungs such as asthma (a common long-term inflammatory condition of the lungs) and chronic obstructive pulmonary disease (or COPD, which is a progressive obstructive lung disease).
Beta2-Adrenoreceptor agonists cause smooth muscle fibres to relax.
Ok, so these researchers found Beta2-adrenoreceptor agonists reduce the production of alpha synuclein in their screening experiment?
Yes, exactly. The cells treated with Beta2-adrenoreceptor agonists generated less of the Parkinson’s associated protein alpha synuclein.
The researchers next injected some mice with Beta2-adrenoreceptor agonists and then looked at the dopamine neurons in the brain, and they found that those cells also produced less alpha synuclein. Interestingly, they also noted that in genetically engineered mice which did not produce any Beta2-adrenergic receptors, the levels of alpha synuclein in the brain were twice as high as normal.
Interesting. But is all of this relevant to humans?
Well, having established that Beta2-adrenergic receptor activation reduces alpha synuclein levels, the researchers next wanted to turn their attention to what is happening in people that have been treated with Beta2-adrenoreceptor agonists. To do this, the researchers used the Norwegian Prescription Database which contains the complete records of all prescribed drugs dispensed at pharmacies to every individual in Norway since 2004 (population: 5 million!).
 Norway. Source: Go-today
Norway. Source: Go-today
They decided to look for prescriptions of the Beta2-adrenoreceptor agonist, Salbutamol to see whether use of this drug could reduce the risk of developing Parkinson’s. Amazingly, they found that Salbutamol was associated with decreased risk of developing Parkinson’s – literally, a 1/3 reduction in risk (in a dose dependent fashion).
And after noting this, the researchers asked the reverse question: does blocking Beta2-adrenergic receptor (with an antagonist drug) increase one’s risk of developing Parkinson’s?
And guess what they found?
Drum roll please!
Propranolol is often called a ‘beta-blocker’ because it blocks the Beta2-adrenergic receptor. This Beta2-adrenoreceptor antagonist is commonly used to treat high blood pressure and essential tremor.
In their analysis, the researchers found that Propranolol was associated with a two-fold increase in the risk of developing Parkinson’s! This result suggested that Beta2-adrenergic receptor antagonists could be increasing the risk of Parkinson’s.
 Source: PMC
Source: PMC
As you can see from the image above, those individuals who took a high defined daily doses (DDD) of Salbutamol (the red line on the left graph) had a lower risk of developing Parkinson’s than those who were administered low levels of Salbutamol (the green line – which does not differ from the general population level of risk (blue line)). And on the graph on the right the 9,339 individuals treated with Propranolol (green line) had a higher risk of developing Parkinson’s than the general population.
NOTE: It should be stated here that the epidemiological findings of the data from Norway has been the subject of ferocious debate, with research groups reporting that they do not find the same trends in independent datasets (Click here and here to see some examples). Despite this, there have been preclinical data of adrenoceptor agonists in models of Parkinson’s that supports the drug screen findings of the study (Click here and here to read some examples).
Salbutamol does cross the ‘blood-brain barrier’, but not very well. In rodents, only about 5% of the amount of the blood amount reaches brain (Click here to read more about this). Thus, a new brain-penetrant adrenoceptor agonist would need to be developed in order to really test if this class of drugs could be useful as a treatment for Parkinson’s.
Enter Curasen.
|
# # RECAP #2: Molecules that act like the neurotransmitter norepinephrine – called adrenoceptor agonists – have been shown to rescue models of Alzheimer’s and Parkinson’s. Adrenoceptor agonists have also been reported to reduce the production of the Parkinson’s associated protein alpha synuclein and may also reduce the risk of developing the condition. # # |
So Curasen is developing brain-penetrant adrenoceptor agonists?
Yes, and recently the company announced that they have dosed the first volunteer in their Phase I clinical trial of a selective adrenoceptor modulator called CST-2032 (Source).
Phase I studies are the first step in the clinical development of a new treatment, and they assess the safety of an agent in humans. This particular Phase I trial will include approximately 70 healthy volunteers and individuals with different types of neurodegenerative diseases, including Parkinson’s. Up to 11 cohorts are planned for this study, with approximately 4-8 participants being recruited to each cohort.
The study is being conducted in four parts:
- Single increasing doses of CST-2032 in healthy volunteers, with the dose amount increasing across groups (up to 5 dose groups).
- Evaluation of food effect – single fasted and fed doses of CST-2032, in healthy volunteers (1 dose group).
- Multiple increasing doses of CST-2032 or matching placebo in healthy volunteers (up to 4 dose groups).
- Single dose of CST-2032, in adults with mild cognitive impairment or Parkinson’s (1 dose group).
The participants will be assessed using a variety of biomarker assessments, including neuroimaging and autonomic function tests. Depending on the results of the first dosing groups, participants may also receive a low dose of a drug called nadolol with each dose of CST-2032. Nadolol blocks heart rate increases that have been previously seen with beta agonists (such as salbutamol).
This study is taking place in both New Zealand (32 pariticipants) and Belgium. If all goes well, Curasen will be looking to initiate Phase II testing in 2021.
And CST-2032 does not appear to be the only adrenoceptor modulator that Curasen has in development. They have three other drug candidates: CST-101, CST-103, and CST-109. And CST-103 is already in clinical studies in Europe (Click here to read more about clinical trial).
Sounds interesting. But why do you think this is important?
The development of selective adrenoceptor modulators by Curasen represents a novel class of drugs for the potential treatment of Parkinson’s – and the company obviously has an interest in this condition as they are including individuals with Parkinson’s in their Phase I safety study.
I find this an exciting development as we need to be exploring new targets and avenues rather than simply putting all our eggs into the “alpha synuclein” basket.
I also like the shift away from the focusing solely on dopamine neurons. Parkinson’s is a complicated syndrome that involves many different cell types in the brain (and the periphery), and it could be argued that our previous obsession with dopamine neurons has been a limiting factor in advancing new approaches towards modifying the trajectory of the condition(s).
There is also solid reasons why adrenoceptor modulation might be useful in slowing the progression of Parkinson’s. Inflammation is a common feature of PD, and norepinephrine (NE) has been reported to have anti-inflammatory properties. Reductions in NE from the locus coeruleus may lead to increased inflammation and microglial activation, which could be playing a role in the progression of the condition (Click here to read a good review about this).
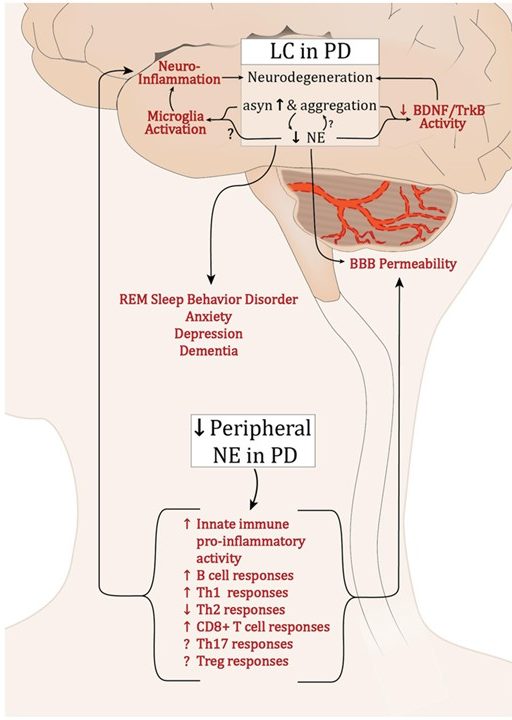 Source: Frontiers
Source: Frontiers
So what does it all mean?
A biotech company called Curasen has recently initiated clinical trials evaluating a new class of experimental drugs for Parkinson’s. Preclinical data indicates that selective adrenoceptor modulators may have beneficial properties for neurodegenerative conditions. These new clinical trials represent a shift in Parkinson’s research as they move the attention away from the long held focus on dopamine neurons and bring the much neglected noradrenalinergic neurons under the spot light.
It will be interesting to see if others follow this trend. Perhaps in 2021 we will learn of additional examples of selective adrenoceptor modulators being tested in the context of Parkinson’s.
We will be keeping a close eye on the progress of the Curasen trials here at the SoPD. Fingers crossed it all goes safely and well.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, Curasen have not requested that this material be produced, nor has the author had any contact with the company or associated parties. This post has been produced for educational purposes only and because the author thought that readers might find it interesting.
The banner for today’s post was sourced from Curasen.


Thank You
LikeLike
Thank you Scott – glad you liked it.
LikeLike
The Mittal et al. 2017 paper does not seem to list the 35 compounds 😦 … is the list available anywhere?
What would the overall effect of high or low dietary sodium intake be on beta adrenergic receptor activation? (I’m trying to figure out why high sodium diet does not seem to make people with multiple system atrophy worse since it seems like it would cause problems due to increased neuroinflammation.)
Interesting coincidence – yesterday I listened to a podcast by Dr Greger on metabolic rate and drinking water which touches on the diving reflex and so of course I am wondering on what effect just drinking water has (people with OH are also advised to drink copious amounts of water).
Link to podcast: https://youtu.be/HyuXNzLzKYM
How about splashing cold water on the face (Wim Hof)?
LikeLike
Hi Rhyothemis,
Thanks for the interesting comment. I can’t find any link to the list of 39 molecules in the manuscript (the supplemental files contains all of the screened agents). You may need to contact the authors directly to find out what the others were (they may of course be working on some of the other molecules). Not sure about the high or low dietary sodium matter. I’ll have a look into it.
Kind regards,
Simon
LikeLiked by 1 person
Thanks for your reply – and I should have thanked you in my initial comment since I am really, really having a good geek out on it.
Something that might possibly relate to the ‘sodium paradox’- in a presentation by Ruslan Medzhitov on reduction of influenza mortality by glucose – he states that part of the mortality reduction effect was due to sodium. Also that death is due to autonomic failure ( specific brain region is affected by ER stress)https://youtu.be/ZmGyf7fv430
Or maybe high sodium diet increases HSP70, or perhaps it has something to do with vitamin C cotransporter function … or something to do with a-syn expression reduction thru cytoskeletal changes – very speculative but it relates to a-syn maybe having anti-fibrotic propertieshttps://www.nature.com/articles/s41467-020-15732-9 and HSD can induce fibrosis – but I think anti-fibrotic flavonoid consumption is associated with lower PD risk …
as you can see, this this has been bugging me for months now …
Have a Happy New Year!
LikeLike
Extremely interesting. I believe that shortly Cambridge University are going to be working on noradrenaline .
Keith
LikeLike
Thanks Keith – glad you liked the post. I hope all is well. I’ll look into the Cambridge research.
Kind regards,
Simon
LikeLike
In the article above, there’s the following paragraph:
This Beta2-adrenoreceptor antagonist is commonly used to treat high blood pressure and essential tremor (please note this second condition – it will be important in our discussion further below).
Unless I’ve missed it, I can’t see another reference to essential tremor. I’m just curious about what the link is and why it’s important?
LikeLike
Hi M,
Thanks for noting this. It was supposed to be a reference to a chunk of text further down in the post that was removed to save space (and reader sanity).
The debate surrounding the replication of the Norway epidemiological data partly centres around correcting the data for tremor – since propranolol is used to treat tremor. When other independent researchers adjusted their data to take account of the treatment of tremor (during 18 months prior to diagnosis), they found no association between propranolol use and Parkinson’s (odds ratio = 0.94 (95%=0.80–1.11)). This detail brought into question some of the early data. But others have replicated the Norway findings AND adjusted for tremor, so it is a rather complicated business to resolve… and to explain in layperson terms on this blog. Hence the reason for removing it.
I hope this makes sense.
Kind regards,
Simon
LikeLike