|
# # # # For a long time a regular request from SoPD readers has been to provide an overview of the clinical trial landscape for Parkinson’s, particularly in the area of drug development. Such projects are difficult, however, as the landscape is broad and dynamic – lots of different approaches being applied and new entrants continually entering the arena. These are positive features, but to characterise the whole field is beyond my simple cognitive abilities. But recently three Parkinson’s research advocates (with help from the research department at The Cure Parkinson’s Trust) tackled this challenge, and the output of their efforts was published in the Journal of Parkinson’s disease in July of this year. In today’s post, we will discuss advocacy, review the current clinical trial pipeline for Parkinson’s, and explore how an analysis of this pipeline could be improved. # # # # |
 Raymond Carver (Source)
Raymond Carver (Source)
“He understood that it took only one lunatic and a torch to bring everything to ruin”
– Raymond Carver
I enjoy old Raymond Carver short story collections (his 1983 ‘Cathedral‘ in particular).
He is not for everyone, but I like him. Particularly ‘What We Talk About When We Talk About Love‘. It is a story about two couples sitting at a kitchen table, drinking gin, and trying to describe what is meant by love.
 Source: Encorespotlight
Source: Encorespotlight
I thought of this short story last year when I was asked during a Q&A session at a support group meeting, “What do we mean when we talk about advocacy?” (that was the exact wording).
I didn’t mention Carver in my answer. Rather I listed some of the various ways that people can become advocates for Parkinson’s. And there are many, and it really depends on what you want to do and what skills you have or want to learn (we will come back to this near the bottom of today’s post).
 Source: Endslaverynow
Source: Endslaverynow
Advocacy comes in many forms. And in today’s post I’d like to share one inspiring example of advocacy.
Earlier this year I played a small role in a wonderful project led by a team of Parkinson’s research advocates who were focused on trying to provide an overview of the clinical trial pipeline for therapies for Parkinson’s, with the goal of raising awareness within the PD community.
The results of their efforts were published in July.
What did they find?
Before we get to that, let me do some introductions. Three advocates were involved with the project.
First, there was Dr Kevin McFarthing. A biochemist by training, Kevin had an impressive career in drug development, including 17 years at Reckitt Benckiser (where he held senior R&D roles, including Global Head of Strategic Alliances). When he was diagnosed with Parkinson’s, he turned his attention to the ongoing drug development efforts for the condition and now maintains the Hope List – a wonderful resource listing much of the clinical and preclinical work being conducted on PD.
Here is a video of Kevin presenting the Hope list in 2019 (if you do nothing else today – watch this video):
Next, there is Sue Buff:
 Sue is a Parkinson’s care partner and research advocate living in the San Francisco Bay Area. Her career background is in software product management, and she maintains the PDTrialTracker website – a comprehensive database that provides “Parkinson’s patients, families, researchers, health care providers, and other interested members of the PD community with analysis of ongoing PD clinical trials and observational studies throughout the world“.
Sue is a Parkinson’s care partner and research advocate living in the San Francisco Bay Area. Her career background is in software product management, and she maintains the PDTrialTracker website – a comprehensive database that provides “Parkinson’s patients, families, researchers, health care providers, and other interested members of the PD community with analysis of ongoing PD clinical trials and observational studies throughout the world“.
And then there is Gary Rafaloff:
 Gary is quite simply an “super advocate” for Parkinson’s. He has:
Gary is quite simply an “super advocate” for Parkinson’s. He has:
- been involved in multiple clinical trials, both as a participant and helping with the planning and management (most recently as a member of the steering committee for the Nilotinib (NILO-PD) trial),
- been an ambassador for Fox Trial Finder,
- testified in front of a FDA panel discussing the need for new and better treatments for Parkinson’s,
- been a consultant to pharmaceutical companies on their patient-centric goals and policies and served on the advisory council for two pharmaceutical companies,
- been a member of the advisory council for PatientsLikeMe (the largest personalized health network in the world, which included over 17,000 members with Parkinson’s),
- And on top of all that, he’s a genuinely nice guy.
The three of them got together at the 2019 Linked Clinical Trials meeting (Click here to read an SoPD post about that), where the idea of an annual report on the clinical trial landscape for Parkinson’s was first discussed.
They next asked Prof Patrik Brundin – one of the editors of the Journal of Parkinson’s – whether he thought there would be any interest from the Parkinson’s research community in such an annual report.
 Prof Patrik Brundin. Source: Mlive
Prof Patrik Brundin. Source: Mlive
Prof Brundin’s answer was immediate: “There would DEFINITELY be interest in that kind of a report“.
So with a little bit of help from the research department at The Cure Parkinson’s Trust, the team began having weekly online meetings to analyse and break down data that they had collected from the ClinicalTrials.gov website.
What is the Clinicaltrials.gov website?
It is a database of both privately and publicly funded clinical studies that are being conducted around the world. At the time that this post is being written, there are 359,682 research studies listed on the database, and they have been conducted in 219 countries around the world. Maintained by the U.S. National Institutes of Health, the Clinicaltrials.gov website was made pubic on the 29th February 2000, and it is the largest, most comprehensive source of data for ongoing clinical trials in the world.
 The team of advocates used data from the ClinicalTrials.gov website and in July the results of the teams analysis on the clinical trials for Parkinson’s were published in the Journal of Parkinson’s.
The team of advocates used data from the ClinicalTrials.gov website and in July the results of the teams analysis on the clinical trials for Parkinson’s were published in the Journal of Parkinson’s.
Here is the report:
 Title: Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020.
Title: Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020.
Authors: McFarthing K, Buff S, Rafaloff G, Dominey T, Wyse RK, Stott SRW.
Journal: J Parkinsons Dis. 2020;10(3):757-774.
PMID: 32741777 (This report is OPEN ACCESS if you would like to read it)
In this study, the investigators downloaded all of the available information from the ClinicalTrials.gov website regarding clinical trials for Parkinson’s that were active between January 2019 and January 2020. The downloaded dataset included a total of 193 trials. To simplify their task, the team decided to focus their attention on drug trials specifically and they decided to template their assessment on a similar report investigating Alzheimer’s clinical trials (Click here to read that Alzheimer’s report).
NOTE: While there is considerable interest in non-drug related studies (such as exercise and diet), the categorisation and analysis of these kinds of studies was found to be rather complicated, so to keep things simple in this first effort the team focused on drug trials.
After they removed all of the observational and non-drug related studies, they were left with 145 active.
They started their analysis by dividing the clinical trials based on whether the agent being tested was considered symptomatic or disease modifying.
What does that mean?
Symptomatic therapies are treatments that address the features of a condition (such as symptoms) without dealing with the underlying biology of the condition. They do nothing to alter the long-term trajectory of the disease, they simply offer temporary relief from the symptoms.
Parkinson’s is a progressive condition, meaning that the symptoms are going to get worse over time. The normal trajectory of the condition looks something like this the image below. The initiation of symptomatic treatments (such as L-dopa – the red line in the image below) bring relief from the symptoms, but they do not alter the overall trajectory of the disease:
 A disease modifying treatment is a therapy that alters the underlying progression of a medical condition, shifting the overall tracjectory of the disease. It is an agent that can slow, stop or reverse the progression of a condition.
A disease modifying treatment is a therapy that alters the underlying progression of a medical condition, shifting the overall tracjectory of the disease. It is an agent that can slow, stop or reverse the progression of a condition.
In the image below, a disease modifying treatment would change the downward trajectory of progression to one of the dotted red lines:
 It does not need to be said, but there are currently no disease modifying treatments approved for Parkinson’s.
It does not need to be said, but there are currently no disease modifying treatments approved for Parkinson’s.
This video provides an interesting presentation by Dr. Kelly Mills from Johns Hopkins who disecusses the difference between symptomatic or disease modifying therapies at great length:
In their analysis, the investigators found that 88 of the 145 trials were focused on symptomatic relief while the remaining 57 trials were considered disease modifying – aimed to slow, stop or reverse the long-term progression of Parkinson’s.
Next the team separated the agents being tested in the clinical trials into 14 different categories based on the the target or mechanism of action of the agent.
What does mechanism of action mean?
In biology, the mechanism of action (MOA) refers to specific biochemical interactions via which a substance produces its pharmacological effect. It usually involves a specific molecular target that the drug binds to, such as an enzyme or receptor. Some agents have multiple mechanism of action, involving several biological pathways.
The team determined 14 categories for their analysis:
- Antioxidants – reducing oxidative stress
- Botanicals – herbal extracts
- Cell therapy – cell transplantation or peripheral delivery of cells.
- Dopaminergic symptom relief – agents that mimic the chemical dopamine.
- Energy and mitochondria – restoring mitochondrial function.
- GBA – enhancing the activity of glucocerebrosidase.
- GLP-1 agonists – a class of diabetes drugs.
- Immunotherapy – antibody-based agents.
- Kinase inhibitors – blocking certain kinase activity.
- Microbiome/GIT – focused on the gastrointestinal tract.
- Neurotrophic factors – delivery of GDNF or CDNF.
- Non-dopaminergic symptom relief – symptomatic therapies not involving dopamine.
- Targeting alpha synuclein – agents inhibiting alpha synuclein aggregation.
- Other – where there was only one treatment for a specific MOA.
And finally, the trials were divided into their respective Phase of clinical testing.
What does that mean? What is a Phase of clinical testing?
Good question.
There are typically 3 Phases involved with getting a new treatment approved for clinical use and they apply to almost all medical conditions.
Once a compound/treatment has been identified, validated in various preclinical models of a medical condition, and a basic phamacological profile has been established in animals (this involves determining if the treatment is toxic and investigating how long the treatment lasts in the body of a lab animal), then an application to health regulators will be made to start testing the treatment in humans.
The first test in humans is called a Phase I clinical trial.
 Source: Closerlookatstemcells
Source: Closerlookatstemcells
The goal of Phase I trials is to determine if the drug is safe. Efforts will also be made to assess the ideal dose for further clinical testing. Phase I trials will often be in healthy individuals (perhaps 20-50 people), involve a single or multiple doses of the treatment, and they are usually very quick (think weeks or a few months).
If the treatment is found to be safe in Phase I, the investigative team will shift their efforts to a Phase II trial.
 Source: Closerlookatstemcells
Source: Closerlookatstemcells
The goal of a Phase II clinical trial is to determine if the drug is safe in your patient population of interest and attempt to provide proof of efficacy (that is, try to provide evidence that the treatment is doing what it is supposed to). Phase II trials usually involve 50 – 100 individuals being on the treatment for a long period of time (they will last approximately 12 months in Parkinson’s, although this varies).
If the treatment is found to be safe and displays evidence of having an effect in Phase II clinical trials (an example of this in Parkinson’s is the Exenatide study – Click here and here to read old SoPD posts about this), the investigative team will then set up for a large Phase III clinical trial.
 Source: Closerlookatstemcells
Source: Closerlookatstemcells
The goal of a Phase III trial is to determine the long-term safety and efficacy of the agent in a large group of the patient population of interest (200+ participants). These are huge, expensive trials that will last 1-2 years on average in the case of a slowly progressing condition like Parkinson’s. Positive Phase III results are required by health regulators in order for an agent to be approved for clinical use.
When the team of advocates looked at the different phases of clinical development, they found that:
- 37 (26% ) trials were Phase 1
- 14 (10% ) were Phase 1/Phase 2
- 61 (42% ) were Phase 2
- 5 (3% ) were Phase 2/Phase 3
- 28 (19% ) were Phase 3
As you will see from the list above, some of the clinical studies bridged two different phases. To heep their analysis simple the trials that bridged phases were shifted into the lower of the two phases (for example, a Phase 1/2 was considered Phase 1).
All of this preparation (determining symptomatic vs disease modifying, categorising the agents, and assigning Phase of testing) then allowed the team to start conducting some statistics and visualising the data in graphs, such as an overview of the entire 145 agents in clinical trials for Parkinson’s:
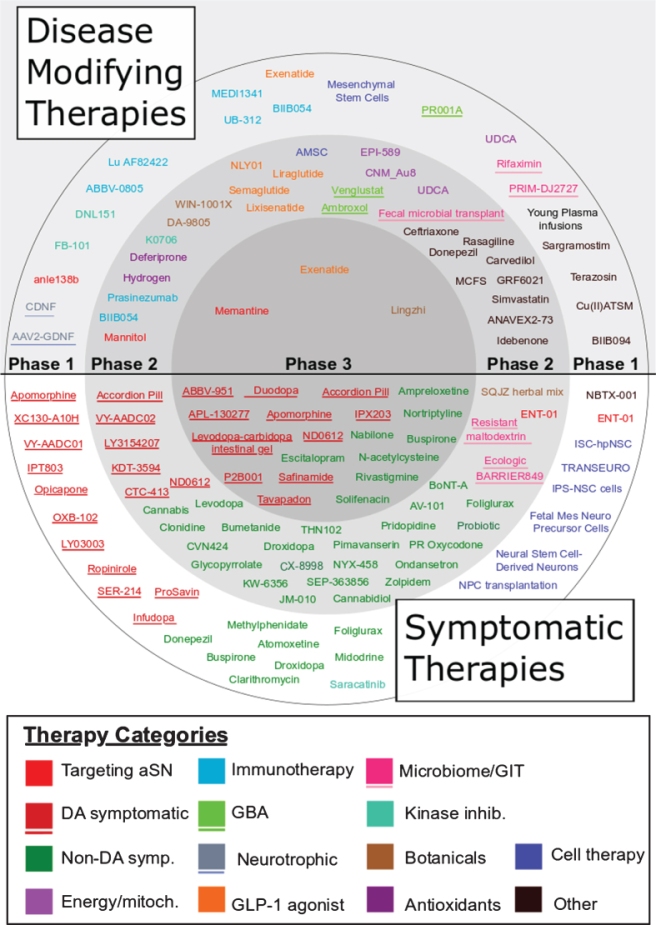 Agents in trial for PD in 2019. Source: JPD
Agents in trial for PD in 2019. Source: JPD
They were also able to break the data down and look at specific phases of clinical trials, such as this graph of Phase I trials:
 Source: JPD
Source: JPD
Although this was just a snap shot of one particular year of clinical trial activity, a worrying aspect of the data was that only 3 clinical trials were being conducted in Phase III for disease modifying therapies. We need to increase that if we are going to have novel therapies being approved by regulators for clinical use.
The team also analysed other aspects of the clinical trial process, such as:
- the percentage of trials evaluating repurposed agents (34% of trials)
- sponsor/collaborator information (industry is involved in 63% of trials)
- target enrollment (16,023 people were involved across all of the trials)
- international regions (majority of these studies were in the USA and Europe)
And the research department at the Cure Parkinson’s Trust was pleased to note that more than 10% of all the disease modifying trials being conducted worldwide are being funded by CPT.
Not bad for a small research charity of 22 employees!
NOTE: The author of this blog is an employee of the Cure Parkinson’s Trust and may be slightly biased (#ShamelessSelfPromo).
If you are interested in reading the full report, it is OPEN ACCESS on the Journal of Parkinson’s website (Click here to read it).
The take home summary of the report, however, is that there is a broad pipeline of therapies currently being tested in clinical trials for PD (both symptomatic and disease modifying). It will be interesting to see how this changes and evolves with future annual reviews.
Excellent stuff. So there going to be another report of clinical trials for 2020-2021?
Yes, the goal is for this to be an annual report.
And part of the reason for writing this post is to ask readers if there are certain aspects of the clinical trial pipeline that they would like to learn more about from such a report. Are there ways that the report could be improved?
One current thought is to improve the layperson dissemination of the information and the team is currently exploring ways in which that could be done.
But they would be keen to hear from others as to what they would like to see in such a report. So please have a read of the report and feel free to leave any thoughts/ideas in the comments section below or contact me directly.
That’s a great example of advocacy, but how else can people advocate for Parkinson’s?
As we mentioned above, advocacy comes in many forms and it really depends on what each individual wants to do or what skills they have (or would like to learn – I knew little about websites or social media before becoming an advocate in my own small way… and yes, it could be argued that my knowledge of these things has not expanded greatly!).
Advocacy does not necessarily mean fundraising or taking part in a clinical trial. Nor does it mean putting oneself out in the public eye (reviewing layperson summaries of grant applications is a critical service that can be done in isolation).
In the image below various advocacy roles are mentioned (I’ll let your imagination determine what “The hacker” refers to):
 If you can think of any other roles or examples of advocacy that readers might be interested in, please feel free to leave them in the comment section below.
If you can think of any other roles or examples of advocacy that readers might be interested in, please feel free to leave them in the comment section below.
So what does it all mean?
There are lots of inspiring stories in the world of advocacy. It would be nice if someone would take the role of Raymond Carver and write some short stories about many of them (the world feels like it needs such positive stories). One their first ports of call in those stories could be Mike Lloyd who runs marathons despite having Parkinson’s… and being blind!
 Mike Lloyd – legend. Source: Newshub
Mike Lloyd – legend. Source: Newshub
Raised in New Zealand, Mike was born with retinitis pigmentosa, a rare, genetic disorder that involve a loss of cells in the retina, resulting in the deterioration of sight. By age 30, he was unable to read printed text. Following the complete loss of vision, Mike joined Achilles International – an organisation that helps people with disabilities compete in mainstream sports. He has been an annual finisher of the New York marathon since 2007, despite being diagnosed with Parkinson’s in 2012 (learn more about Mike by clicking here).
For Mike, it is never about what you can’t do, only what you can do. Plus, he believes passionately that there is no such thing as failure.
Another wonderful example of advocacy is a small team of individuals seeking to inform the Parkinson’s community about what the clinical trial landscape looks like for the condition. The fruit of their efforts, described in this post, illustrates not only that there is a robust pipeline of novel therapies being clinically tested for Parkinson’s, but also that there are numerous ways in which everyone can have a role in improving things.
It is never about what you can’t do, only what you can do.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE – The author of this post is an employee of the Cure Parkinson’s Trust, so he might be a little bit biased in his views on the topic discussed. The trust has not requested the production of this post, but the author considered it interesting and important to share with the Parkinson’s community.
The banner for today’s post was sourced from ieee



I suppose the most promising drugs in stage 3 can be gleaned from looking at the topics on this website over the past year or so.
Thanks – overviews are very helpful to me.
LikeLike
Why no mention of PR001 from Prevail
LikeLike
Makes me wonder what implications PR001 may have for idiopathic Parkinson’s disease, and not just GBA type Parkinson’s.
LikeLike
I wouldn’t think any because you don’t have the gene mutation. That said apparently there’s a new company out of Chicago that is in pre-clinical stage with developing a pill that gets the brain to manufacture G case. That said I’m a lawyer and totally not a doctor.
LikeLike
Nevermind! Couldn’t see.
I do have a follow-up question though for disease modification. Can it really ever reverse what is happened to your brain? My understanding was that you could hold progression, but you could probably not reverse damage.
LikeLike
Certainly a useful overview of many things that are going on. It would help to be able to link to topics/studies of special interest to explore them more thoroughly (or have I missed the linking?) The interim reports after Phase I and II would also be of interest.
LikeLike
What is involved in “layperson reviews of grant applications”?
LikeLike
Simon, a really useful post – particularly as someone who struggles with the science. Must have taken you ages (as do all of these, I guess). Many many thanks for all you do and for this blog.
LikeLike
How are you doing jellywoman? I read your blog from time to time. Are you trying to be a part of ambroxol phase 3?
LikeLike
I am doing in Ambroxol on my own in the US. I’ve been doing it for about nine months and haven’t seen any difference that I have noticed. I am very early stage.
LikeLike
Thanks for the excellent article as usual. What would be really useful is if we had some progress updates on the trials..ie. finished recruiting.. trial ended..peer review ongoing. Its so annoying to see trials are never updated with accurate information or dates.. a little bit of hope can go a long way.
LikeLike
I have great difficulty in envisaging any trial of a single agent slowing progression of idiopathic, age-related PD. whether gut-first or brain-first. The one intervnetion that should depress inflammation , combat oxidative stress, improve calcium regulation is conspicuously absentr from horizon scanning deliberations. Since most populations are chronically badly deficient this hormone may well be of central importnace in the etiology of i PD.
Yet D3 is ignored. Why?
LikeLike
I would argue that biib 094 is a candidate targeting alpha-synuclein. It basically interferes with protein transcription. It was developed by ionis
LikeLike
Thanks as always Simon. Also thanks to Sue and Gary. My participation in a trial was 100% attributable to their ongoing efforts.
LikeLike
I’m feeling frustrated with all the articles, science and data about medications deemed safe, capable of rapid repurposing, over-the-counter access currently available for Parkinson’s that patients can’t get.
Who is advocating for us?
LikeLike