|
# # # # 2020 has been a dreadful year for most of the world – burdened by the outbreak and consequences of COVID-19. Despite this, there has been a steady stream of biotech acquisitions related to Parkinson’s which have helped to keep morale high in the PD research community. In October alone, we saw the Portuguese pharmaceutical company Bial purchase GBA-associated Parkinson’s biotech firm Lysosomal Therapeutics (Click here to read more about this) and the acquisition of the inflammasome-focused biotech firm Inflazome was being bought by Roche (Click here to read more about this). Today brought news of yet another pharmaceutical company – this time Eli Lilly – purchasing a Parkinson’s-focused biotech company (Prevail Therapeutics). In today’s post, we will explore what Prevail Therapeutics does, why Eli Lilly might be so interested in this company, and why it could be an encouraging move for individuals with a sub-type of Parkinson’s. # # # # |
 Colonel Eli Lilly. Source: SS
Colonel Eli Lilly. Source: SS
The civil war veteran, Colonel Eli Lilly started his pharmaceutical career in a drug store in Greencastle (Indiana) in 1869.
Several years later (in 1873) he shifted into the manufacturing of pharmaceuticals (in association with John F Johnston). Two years after that, Lily disolved their partnership, sold his assets, and used the proceeds to set up “Eli Lilly and Co” in Indianapolis.
 Source: Wikimedia
Source: Wikimedia
He started the company in a rented building on the 10th May, 1876. He was 38 years old, with working capital of $1400 and just three employees. The first medicine that he produced was quinine – a drug used to treat malaria.
Since that humble start, the company (now more commonly known as just “Lilly”) has grown to become one of the 20 largest pharmaceutical companies in the world (Source), with offices in 18 countries and products sold in 125 countries (Source).
 Lilly was the first company to mass-produce the polio vaccine and it was also one of the first pharmaceutical companies to produce human insulin using recombinant DNA. Lilly is currently the largest manufacturer of psychiatric medications, including Prozac (Source).
Lilly was the first company to mass-produce the polio vaccine and it was also one of the first pharmaceutical companies to produce human insulin using recombinant DNA. Lilly is currently the largest manufacturer of psychiatric medications, including Prozac (Source).
Today, the company employs approximately 38,000 people worldwide, and operates through two key business divisions:
- Human Pharmaceutical Products, which involves the production and sale of prescription medications in the fields of endocrinology, oncology, cardiovascular health, and neuroscience
- Animal Health Products, comprising the development and sale of treatments for domestic and farm animals
This is all very interesting, but what does any of it have to do with Parkinson’s?
This week the biotech world was alerted to the news that Eli Lilly was purchasing a biotech company that is focused on developing a novel treatment for a subtype of Parkinson’s.
That company is called Prevail Therapeutics.
 What does Prevail Therapeutics do?
What does Prevail Therapeutics do?
Prevail Therapeutics is a gene therapy company focused on neurodegenerative conditions, particularly GBA-associated Parkinson’s.
What is GBA-associated Parkinson’s?
GBA is a gene – a specific section of DNA – that provides the instructions for making a protein called glucocerebrosidase (or GCase). GCase is an enzyme that helps with the digestion and recycling of various proteins (particularly glucocerebrosides) inside cells.
Genetic variations in the GBA gene are associated with a condition called Gaucher disease (Click here to read a previous SoPD post on GBA and Gaucher disease).
In the 1990s, physicians began to notice that patients with Gaucher disease appeared to have a higher risk of developing Parkinson’s. An example of this was a report published in 1996 that described individuals with Gaucher disease who also exhibited an early-onset, severe form of Parkinson’s with a greater element of cognitive decline:
 Title: Occurrence of Parkinson’s syndrome in type I Gaucher disease.
Title: Occurrence of Parkinson’s syndrome in type I Gaucher disease.
Authors: Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, Reches A, Bembi B, Zimran A.
Journal: QJM. 1996 Sep;89(9):691-4.
PMID: 8917744 (This article is OPEN ACCESS if you would like to read it)
In this study, the Israeli researchers reported on 6 people with Type I Gaucher disease (which up until that point had not been considered neuronopathic). All six of the subjects also exhibited the hallmarks of a rather severe form of Parkinson’s, which made its appearance in the 4th to 6th decade of life and displayed an aggressive progression and was largely unresponsive to conventional anti-Parkinson therapy (such as L-dopa).
Larger follow up studies have reported similar cases (Click here and here for an example).
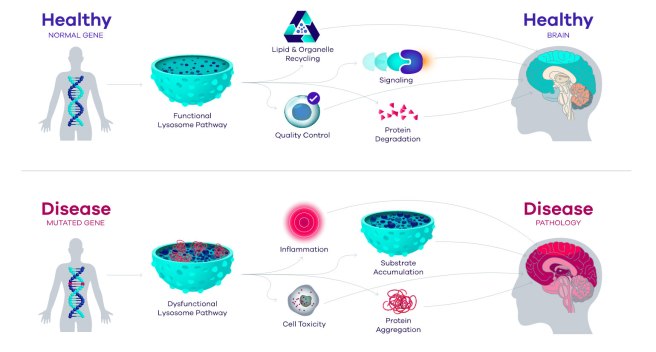
In healthy unaffected individuals, the enzyme glucocerebrosidase (or GCase) helps to break down proteins and helps to maintain proper lysosomal function (lysosomes are small bags inside of cells where the enzyme conducts its job).
 Source: Prevail
Source: Prevail
In individuals with GBA genetic variations, however, the enzymatic breakdown of proteins is disrupted, which results in the build up and accumulation of proteins. This can lead to inflammation and ultimately cell death. And this process is believed to give rise to the appearance of Parkinson’s symptoms in some of these individuals (GBA variants are quite common in the general population, but not everyone with them goes on to get Parkinson’s).
Numreous efforts have been made to identify drugs which may be able to correct this situation, such as the Ambroxol clinical trial program (Click here to read a previous SoPD post on that topic).

Ambroxol. Source: Skinflint
The issue with drug treatments, however, is that the therapy requires the patient to continuously take the drug.
And this is where a gene therapy approach – like what Prevail is proposing – may provide a more convenient solution.
|
# RECAP #1: Prevail Therapeutics is a biotech company developing a gene therapy treatment for GBA-associated Parkinson’s. GBA is a section of DNA (a gene) that provides the instructions for making a protein called Glucocerebrosidase (or GCase), which helps with the digestion and recycling of various proteins. Genetic variations in the GBA gene are associated with an increased risk of developing Gaucher disease or Parkinson’s. # |
What is gene therapy?
Gene therapy is an experimental treatment approach that involves treating medical conditions with DNA rather than drugs.
 Source: Baltimoresun
Source: Baltimoresun
Gene therapy basically involves introducing a new piece of DNA or replacing a faulty piece of DNA within a population of cells. DNA, as you may remember from high school science class provides the instructions for making proteins in the a cell and these proteins are the bits that actually do stuff.
By introducing a new piece of DNA into a cell, the cell can start to produce a functioning protein that it may not normally produce. In some diseases, a cell may normally produce a particular protein, but because the genetic instructions in the DNA (a section of the DNA called a gene) for that protein have a small error (a genetic mutation), a non-functioning version of the protein is actually being produced. The introduction of the new correct (functioning) version of that piece of DNA (or gene) into a cell can start the production of a functional version of the protein.
 Gene therapy. Source: yourgenome
Gene therapy. Source: yourgenome
Taking this approach one step further, we can take sections of DNA that contain the genes involved with the production of a proteins that would be beneficial for the cell, such as GDNF. By then injecting a virus with the DNA for GDNF into the brain, we can produce GDNF in any infected cells (it’s slightly more complicated than that, but you get the basic idea). And such a gene therapy approach is currently being tested in a clinical trial (Click here to read a recent SoPD post about this).

Gene therapy for Parkinson’s. Source: Wiki.Epfl
I’m sorry, but did you say viruses?
Yes, if you remove the viral DNA from inside a virus and replace it with something useful, then a virus becomes a very useful biological delivery system. Far superior to anything we humans have devised thus far. Viruses are easy to produce and manipulate, and they can even be engineered to target specific cell types.

Viruses – an ideal biological delivery system. Source: HuffingtonPost
And these viruses have been engineered NOT to replicate or cause disease. Their ability to replicate themselves has been completely removed.
They deliver the proposed DNA and that is all.
And there is a particular type of virus that Prevail is planning to use in their PR001 treatment.
What sort of virus?
An AAV virus.
What is an AAV virus?
Adeno-associated viruses (or AAV) are a kind of virus that are popular with researchers because A.) they readily infect human and primate cells, and B.) they produce little (if any) immune response, and C.) they are non-pathogenic (they don’t cause any known diseases).
Given these characteristics, AAVs have been used in most of the gene therapy clinical trials thus far:
 A few of the AAV-based gene therapy clinical trials. Source: Wikipedia
A few of the AAV-based gene therapy clinical trials. Source: Wikipedia
They were originally discovered in the preparation of another type of virus, called an adenovirus (hence the name ‘Adeno-associated’). They were believed to simply be a contaminant of that preparation. Further research, however, revealed that AAVs belong to the Dependoparvo genus of viruses, which in turn belongs to the family Parvoviridae.
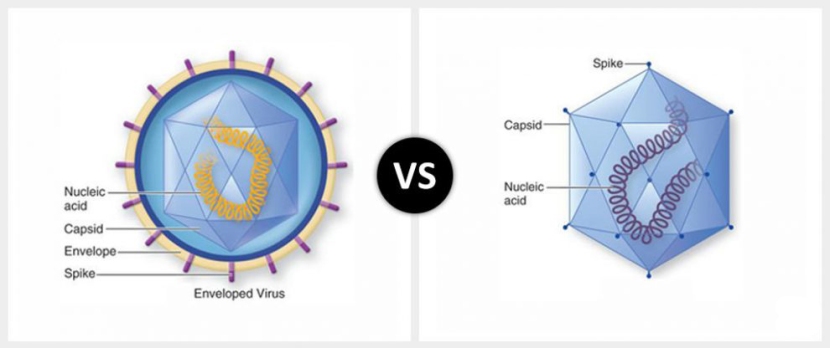
AAVs are single-stranded DNA viruses, and they are one of the smallest viruses (approximately 22 nm in diameter) with a non-enveloped capsid. The capsid is the shell surrounding the genetic material of the virus. Viruses are either enveloped or non-enveloped. “Enveloped” means that a second casing surrounds the capsid, providing further protection for the virus, while “non-enveloped” viruses have only the capsid.
Enveloped (left) vs Non-enveloped (right) viruses. Source: Differencebtwn
Given the reduced amount of casing, non-enveloped viruses are generally more virulent (more infectious) than enveloped viruses (a good example of a non-enveloped virus is the influenza virus). Non-enveloped viruses do not survive outside of an organism for long though.

The AAV capsid. Source: Wikipedia
PR001 is an AAV9 virus.
Eleven different (or serotypes) of AAV have thus far been identified (Source). AAV9 are very effective at infecting cells in the brain (such as neurons – click here to read more about AAV viruses).
Have AAV9 viruses been used before in clinical trials?
Yes, they have.
At the time of writing this post, there were at least 22 registered clinical trials using AAV9 viruses (Click here to read more about them).
In 2017, Prevail entered into an exclusive worldwide license agreement with REGENXBIO to develop and commercialise gene therapy products using REGENXBIO’s NAV AAV9 vector for the treatment of Parkinson’s and other related neurodegenerative conditions. These viruses are remarkably more robust and selective compared to previous generations.
In June 2018, the U.S. FDA granted Fast Track designation to REGENXBIO for RGX-111, which is a novel, one-time investigational treatment for Mucopolysaccharidosis Type I (MPS 1). MPS 1 is a rare lysosomal storage disease caused by deficiency of an enzyme called iduronidase (IDUA). RGX-111 is a NAV AAV9 vector that is designed to deliver the human iduronidase (IDUA) gene directly to cells in the brain (Click here to read the press release).
That trial is now ongoing (Click here to learn more about this study).
So PR001 is an AAV9 virus. But what does it contain?
PR001 will deliver a section of DNA which will provide infected cells with the instructions for making a normal version of the GCase enzyme. AAV viruses integrate their cargo DNA into the DNA of the host cell, meaning that the GCase enzyme can be made continuously over time (better than taking a drug every few hours right?).
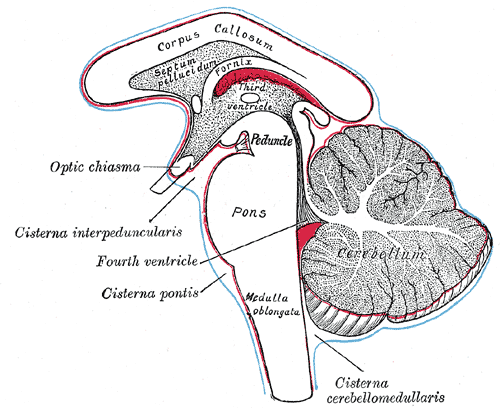
The intended route of delivery for PR001 is via a single injection into the intra cisterna magna.
What is the intra cisterna magna?
Our brains sit in a solution called cerebrospinal fluid, and there are areas of our brains where this liquid can be accessed by doctors. The intra cisterna magna is one of these areas. It is at the base of our skulls (under the cerebellum of the brain), and a needle can be inserted at the back of our heads, near the top of the neck, to access it.
The cisterna magna is labelled “cisterna cerebellomedullaris” on the lower right side of the image below.
Source: Wikipedia
Cisterna magna delivery does require surgery, but it will not be as long or as invasive as some other experimental gene therapy approaches that have been previously discussed on this website (Click here and here for some examples).
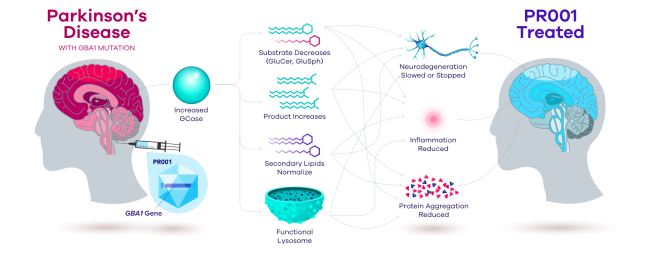
So by injecting a virus – containing the instructions for a normal version of the GCase enzyme – into the fluid surrounding the brain (via the base of the skull), Prevail are hoping to correct GBA-associated Parkinson’s?
That is the plan.
The virus will infect a high number of cells in the brain, and those cells will start to produce normal GCase enzyme and this will hopefully restore normal lysosomal function – in theory making the cells healthier and improving their survival.
 Source: Prevail
Source: Prevail
And this outcome will ideally have a disease modifying effect on the course of the condition, by slowing down or stopping the progression.
As I say, that is the plan. We await the results of clinical trials to find out if it will work.
|
# # RECAP #2: Gene therapy is a method of treating medical conditions using DNA rather than drugs. It typically involves carefully engineering a virus to deliver a specific section of DNA to cells, in order for those cells to start producing the protein associated with that region of DNA. Prevail Therapeutics is using an AAV virus (PR001) to deliver a correct copy of the GBA gene to the brain of people with GBA-associated Parkinson’s to increase GCase enzyme activity in cells. By doing this, the hope is to slow down the progression of the condition. # # |
Is there any evidence to suggest that this approach might work?
I am not aware of any published data on PR001 (and I’m happy to be corrected on this). And most of the publications listed on the Prevail website are related to the clinical aspects of GBA-associated Parkinson’s (Click here to read more about this). And this is not a critique – most biotech companies keep their intellectual property close to their chests.
But when Prevail Therapeutics became a publically traded company, as part of that process, the company had to file papers with the U.S. Securities and Exchange Commission (Click here to read those filing).
 Source: Wikipedia
Source: Wikipedia
In those filings, the company provided a great deal of information about PR001.
We shall review some of that data here – but please note, that this is unpublished data. Being present in a Government document it is in the public domain, but it has not yet been peer-reviewed or published in a scientific journal.
Moving on.
The company used a mouse model of GBA-associated Parkinson’s which involves daily delivery of a compound called conduritol-ß-epoxide (or CBE). CBE is a pharmacological inhibitor of GCase. The mice were subsequently given a single treatment of one of 3 different doses of PR001 (denoted by the number of vector genomes, or vg):
- Low dose of 3,200,000,000 vector genomes, or 3.2 x 109 vg
- Medium dose of 10,000,000,000 vector genomes, or 1.0 x 1010 vg
- High dose of 32,000,000,000 vector genomes, or 3.2 x 1010 vg
All three doses were found to be well tolerated and resulted in the detection of vector genomes in the brain and spinal cord five weeks after delivery. GCase activity, which was reduced in CBE-treated mice, was found to be increased by the highest dose of PR001 in the brain at five weeks. The CBE-treated mice were also found to have raised levels of the toxic glycolipids, GluCer and GluSph, in the brain, which was reduced by PR001 administration in a dose-dependent manner (see image below):
 Source: SEC
Source: SEC
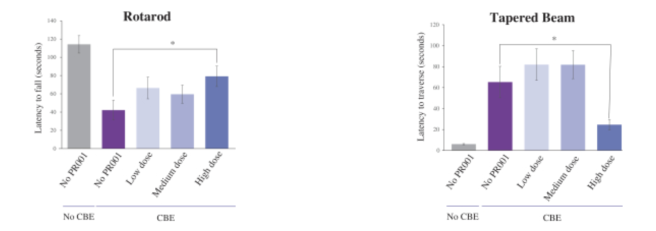
And PR001 was found to have positive impact on the behaviour of the mice. While mice treated with just CBE had reduced performance on the tests of motor function (rotarod and tapered beam), the highest dose of PR001 caused a statistically significant improvement in motor function (see image below):
 Source: SEC
Source: SEC
The company also looked at neuroinflammation in CBE treated mice, and found that CBE induced reactive astrogliosis (as determined by glial scarring) and microgliosis (as determined by a marker called Iba1). Remarkably, PR001 treatment significantly reduced both reactive astrogliosis and microgliosis – in a dose-dependent manner (see image below):
 Source: SEC
Source: SEC
All of the data above came from experiments that only lasted 5 weeks. In a longer-term assessment of PR001 in CBE treated mice, the researchers tested the treatment over a 6 month period and “observed persistent and durable effects” – GCase activity levels remained significantly increased in the cerebral cortex of treated mice, and there was a corresponding reduction of lipid accumulation (see image below):
 Source: SEC
Source: SEC
The researchers also investigated levels of aggregated protein in mice that have been genetically engineered to produce high levels of human alpha synuclein protein which carries the A53T genetic mutation. When these mice were treated with PR001, there was a reduction in levels of alpha synuclein aggregates (see graph below):
 Source: SEC
Source: SEC
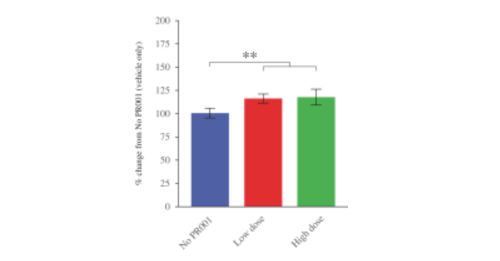
And finally the company conducted PR001 safety and biodistribution testing in healthy non-human primates. While I appreciate some readers will object to this type of experimentation, it is a regulatory requirement before any novel therapy can be tested in humans. In addition, the company “plans to use the safety and biodistribution results from these studies to inform dose selection in our clinical trials“.
The animals were treated with either:
- Control solution
- PR001 low dose (2.1 x 1010 vg/g brain, or vector genomes per gram of brain mass)
- PR001 high dose (8.0 x 1010 vg/g brain)
Six months after PR001 administration, levels of GCase in the brain were found to be significantly raised (see graph below).
 Source: SEC
Source: SEC
The effect in the graph may not look extremely impressive (a 10-20% increase), but remember these animals are normal – they do not have any lysosomal dysfunction or any GCase deficit. In addition, this data suggests that increasing GCase levels does not have a deterimental effect over a 6 month period.
There is a lot more data in the SEC filing for readers who are interested to learn more (Click here to read more about it). In addition, Prevail presented a poster of their preclinical data at the 2020 Alzheimer’s Association International Conference in July (Click here to see that poster).
|
# # # RECAP #3: In unpublished preclinical data, PR001 appears to restore GBA activity in mice treated with a chemical called CBE, which is a pharmacological inhibitor of GCase. This treatment also improves behavioural measures in chronically CBE treated mice. PR001 was found to be safe and well tolerated in normal healthy animals and improved waste disposal function beyond 6-months post administration. # # # |
Has Prevail started clinical testing of PR001?
On the 4th June, 2019, Prevail Therapeutics announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s Investigational New Drug (IND) application for PR001.
This news gave the company a green light to “initiate a Phase I/II clinical trial that will investigate the safety and tolerability of PR001, and will also measure key biomarkers and exploratory efficacy endpoints, in patients with PD-GBA” (Source).
On the 8th July, Prevail received U.S. FDA “Fast Track Designation” for PR001 for the GBA-associated Parkinson’s (Click here to read more about this). Fast Track Designation is a process designed to “facilitate the development and expedite the review of product candidates to treat serious conditions and fill an unmet medical need“.
The first clinical study of PR001 – called the “PROPEL study” – is currently ongoing.
It is a non-randomised, open label Phase I/IIa clinical trial evaluating the safety and tolerability of two doses (low and high) of PR001 in up to 16 individuals with moderate-to-severe GBA-associated Parkinson’s. The trial will also be evaluating the effect of PR001 on biomarkers of disease activity and on Parkinson’s clinical efficacy measures. Most of the measures will be made at 12 months post administration, but the safety follow up will be at least 5 years. The trial is scheduled to finish in 2027 (Click here to read the details of the study).
In January of 2020, the company reported that it is “on track to report interim data on a subset of patients in the second half of 2020″ (Source).
But this was before the Lilly acquisition was made.
So what do we know about the Lilly purchase?
Today (December 15th 2020) Eli Lilly announced a definitive agreement to purchase Prevail Therapeutics for approximately US$1.04 billion (Source).
“The acquisition will establish a new modality for drug discovery and development at Lilly, extending Lilly’s research efforts through the creation of a gene therapy program that will be anchored by Prevail’s portfolio of clinical-stage and preclinical neuroscience assets” (Source).
It sounds as though Lilly is committed to developing PR001 for GBA-associated Parkinson’s. Mark Mintun – vice president of pain and neurodegeneration research at Lilly – is quoted as saying “We look forward to completing the proposed acquisition and working with Prevail to advance their groundbreaking work through clinical development”.
And it is also interesting to note that Lilly also recently purchased another biotech called Disarm Therapeutics.
 In October, Lilly announced that they were acquiring Disarm (Source), who have a preclinical programme developing SARM1 inhibitors for conditions involving axonal degeneration, such as amyotrophic lateral sclerosis (ALS) and multiple sclerosis.
In October, Lilly announced that they were acquiring Disarm (Source), who have a preclinical programme developing SARM1 inhibitors for conditions involving axonal degeneration, such as amyotrophic lateral sclerosis (ALS) and multiple sclerosis.
In addition, in November Lilly signed a deal with Precision BioSciences.
 The collaboration is focused on Precision BioSciences DNA editing technology, with an initial focus on developing therapies for Duchenne muscular dystrophy and two other undisclosed gene targets (Source).
The collaboration is focused on Precision BioSciences DNA editing technology, with an initial focus on developing therapies for Duchenne muscular dystrophy and two other undisclosed gene targets (Source).
So it would appear that Lilly is rather committed to both neurological and gene therapy treatments.
And this suggests that Lilly looks like a good home for Prevail.
Interesting. What else are Prevail doing?
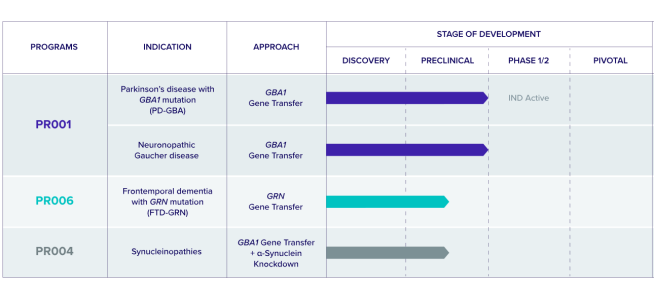
On their website, the company has this table outlining their pipeline of projects:
 Source: Prevail
Source: Prevail
PR001 and PR006 are both in clinical trial now, but the thing that catches one’s eye is PR004. It is described as “an AAV9 vector to deliver the GBA1 gene, which encodes glucocerebrosidase (GCase), and a molecule that suppresses expression of α-Synuclein“.
A combination gene therapy treatment???
I will be interested in learning more about this in the near future.
So what does it all mean?
A relatively young (just over 3 years old) biotech company (Prevail Therapeutics), making significant progress on a novel gene therapy approach (PR001) for a subtype of Parkinson’s (GBA-associated PD), has been acquired by a major pharmaceutical company (Lilly). This news represents yet another example of an ongoing “biotech buy up” trend being undertaken by the pharmaceutical industry.
Concerns about the big pharmas walking away from neurodegeneration less than 2 years ago seem to be drifting away as this year we have seen:
- Biogen tied a knot with Denali (Click here to read an SoPD post about this)
- Bayer acquired Asklepios BioPharmaceutical who then merged with Brain Neurotherapy Bio -the GDNF gene therapy biotech firm (Click here to read a SoPD post about this)
- Bial purchased Lysosomal Therapeutics (Click here to read more about this)
- Inflammasome-focused biotech firm Inflazome was being bought by Roche (Click here to read more about this).
In addition to other less high profile deals.
Gene therapy requires a long term outlook (both in terms of strategy and resources) which the large pharmaceutical companies can bring so it is enormously encouraging to see Lilly entering this space for Parkinson’s. Here at the SoPD, we will be watching to see what updates and guidance the company provides with the future development of PR001 in 2021.
Stay tuned.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: Many of the companies mentioned in this website are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Eli Lilly and Prevail Therapeutics


5 thoughts on “Prevail lands on a Lilly pad”