|
# # # # For a long time it was been reported that coffee may be able to reduce the risk of developing Parkinson’s, but the mechansim by which this association could be occurring has remained elusive. Now researchers from South Korea have discovered a biological pathway that could help to explain the protective association. It involves a protein called PARP and a chemical called chlorogenic acid. In today’s post, we will explore the research suggesting a link between coffee and a lower risk of Parkinson’s, discuss what PARP and chlorogenic acid are, and review the new research that may bring all four topics together. # # # # |

Kaldi the goat herder. Source: CoffeeCrossroads
Legend has it that in 800AD, a young Ethiopian goat herder named Kaldi noticed that his animals were “dancing”.
They had been eating some berries from a tree that Kaldi did not recognise, but being a plucky young fellow – and being fascinated by the merry behaviour of his four-legged friends – Kaldi naturally decided to self-experiment by eating some of the berries for himself.
The result?
He became “the happiest herder in happy Arabia” (Source).
This amusing encounter was apparently how humans discovered coffee. It is most likely a fiction as the earliest credible accounts of coffee-consumption emerge from the 15th century in the Sufi shrines of Yemen, but since then coffee has gone on to become one of the most popular drinks in the world.

Fancy a cuppa? Source: Science-All
Interesting, but what does coffee have to do with Parkinson’s?
A lot.
There is a long association known between coffee and Parkinson’s, and the discovery of that association was made in Hawaii.
 Honolulu. Source: Kuoni
Honolulu. Source: Kuoni
The Honolulu Heart Study was started in October 1965. It involved 8,006 “non-institutionalized men of Japanese ancestry, born 1900-1919, resident on the island of Oahu” (Hawaii). The participants were interviewed and given physical examinations every few years, and over time the study built up a HUGE amount of epidemiological information.
Seriously – as a result of the vast database, the Honolulu Heart Study pops up in medical research for all kinds of age related conditions.
And given the enormous number of individuals involved in the study and the length of time that they were followed, it was inevitable that a certain number of them would go on to develop Parkinson’s as the study progressed. Given this situation, the Honolulu Heart Study represents one of the largest epidemiological studies of Parkinson’s to date.
By 1994, 92 of the participants had been diagnosed with the condition and the researchers were able to look at the longitudinal data and explore which lifestyle features were associated with an increased risk of developing Parkinson’s. By comparing data collected from those with PD to those without, the researchers found that Parkinson’s was inversely associated with coffee intake (meaning the more coffee these Japanese men drank, the less likely they were to be diagnosed with Parkinson’s – click here to read more about this).
 And this data has been replicated in follow-up analyses, such as this report in 2000:
And this data has been replicated in follow-up analyses, such as this report in 2000:

Title: Association of coffee and caffeine intake with the risk of Parkinson disease.
Authors: Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR.
Journal: JAMA. 2000 May 24-31;283(20):2674-9.
PMID: 10819950 (This article is OPEN ACCESS if you would like to read it)
The researchers behind this article analysed the data from the Honolulu Heart Study again in 1998 (with 102 individuals then diagnosed with PD) and (again) they found that the age-adjusted incidence of Parkinson’s declined consistently with increased amounts of coffee intake (from 10.4 per 10,000 person-years in men who drank no coffee to just 1.9 per 10,000 person-years in men who drank at least 28 oz/day (800ml/day)).
This finding indicated to the investigators that higher coffee intake appears to be associated with a significantly lower incidence of Parkinson’s. And subsequent independent studies have replicated this association (Click here for anexample).
Numerous preclinical studies have suggested that the neuroprotective effect may be a result of caffeine (click here for a review on this topic), but a clinical trial found that 6–18 months of caffeine “did not provide clinically important improvement of motor manifestations of Parkinson’s” (Click here to read more about this).
And this has left the research field wondering what particular component(s) of coffee could be having the potential neuroprotective effect on Parkinson’s.
|
# RECAP #1: Coffee consumption appears to reduce the risk of developing Parkinson’s at a later age. Resesarchers have been exploring which component of this popular drink could be having this effect. Some preclinical data points towards caffeine, but a recent clinical trial failed to see any impact on the motor features of Parkinson’s. # |
So if not caffeine, then what could be having this effect?
Coffee is made up of lots of interesting chemicals.
But recently researchers from South Korea have indentified one in particular with an interesting mechanism of action.
They published their research this month:
 Title: Activation oftheAkt1-CREB pathway promotes RNF146expression toinhibit PARP1-mediated neuronal death
Title: Activation oftheAkt1-CREB pathway promotes RNF146expression toinhibit PARP1-mediated neuronal death
Authors: Kim H, Park J, Kang H, Yun SP, Lee Y-S, Lee Y-I, LeeY
Journal: Science Signaling 22 Dec 2020, 13, 663, eaax7119.
PMID: N/A
In this study, the researchers were interested in a protein called PARP.
What is PARP?
Poly (ADP-ribose) polymerase (or PARP) is a family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death. There are currently 17 different types of PARP.
The research discussed in today’s post is focused on PARP1, but for simplicity sake I will only refer to it as PARP.
The main function of PARP in cells is to detect DNA damage and initiate a response.
How does it do that?
When a cell is put under stress, there will quite often be damage to the DNA in that cell.
 Different forms of stress that lead to DNA damage. Source: Sierraoncology
Different forms of stress that lead to DNA damage. Source: Sierraoncology
And given the importance of DNA, mother nature has very wisely devised several very clever systems of DNA repair to maintain the integrity of this precious molecule.
One of those DNA repair mechanisms involves PARP.
When damaged DNA is detected, PARP will bind to the DNA and begin to synthesise chains of poly-ADP ribose (or PAR). These tendrils of PAR serve as a signalling mechanism for DNA-repairing enzymes, making them aware of the damage and recruiting their help to fix it.
 PARP initiating DNA repair mechanisms. Source: bpsbioscience
PARP initiating DNA repair mechanisms. Source: bpsbioscience
This is a very good system if the DNA damage is not too bad and if PARP activity is kept under strict control. But problems start to arise when PARP gets a bit carried away and becomes over activated, causing an accumulation of PAR.
You see, if there is too much PAR floating around with nothing to do, it will start to cause trouble.
Trouble in the form of Parthanatos.
What is Parthanatos?
Parthanatos is a form of controlled cell death – similar to classical apoptosis. Parthanatos is characterised by two main features:
- the excessive production of PAR
- the release of a protein called ‘apoptosis-inducing factor‘ from the mitochondria
What are mitochondria?
Mitochondria are the power stations of each cell. They help to keep the lights on. Without them, the party is over and the cell dies.
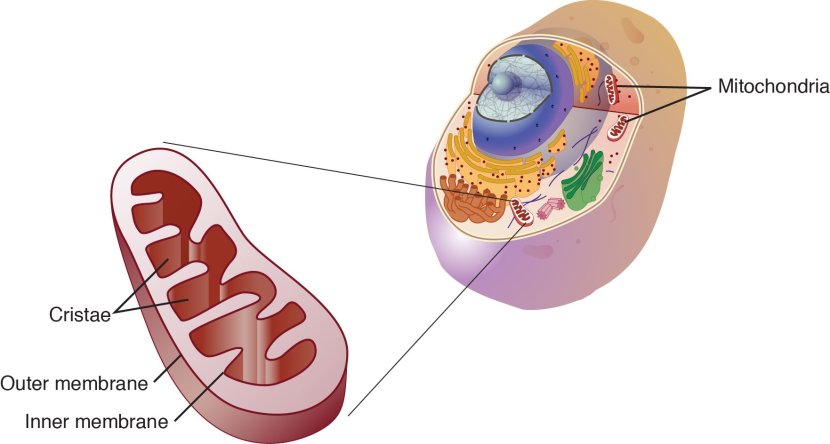
Mitochondria and their location in the cell. Source: NCBI
Mitochondria can also have a powerful influence of cell survival in other ways.
Theses small bean shaped objects not only provide energy for the cell, but they can also release signalling proteins which can instruct the cell what to do.
For example, when PAR reaches the mitochondria it will bind to a protein called apoptosis-inducing factor, which is a protein that (as the ‘label-on-the-can’ suggests) induces apoptosis, a type of programmed cell death.
 Source: Sciencedirect
Source: Sciencedirect
PAR entering the mitochondria causes apoptosis-inducing factor to be released from mitochondria, and once unleashed apoptosis-inducing factor makes its way to the nucleus where it will start to activate regions of DNA that are involved with shutting down and killing the cell in a very clean and organised fashion (Click here to read more about this).
Thus, left unchecked, this process will rapidly lead to the cell dying.
|
# # RECAP #2: PARP (or Poly (ADP-ribose) polymerase) is a protein involved in detecting DNA damage and initiating a repair response. It achieves this by binding to damaged DNA regions and beginning to synthesise chains of poly-ADP ribose (or PAR). But if this process becomes unregulated and too much PAR begins to build up, it can lead to a form of cell death called Parthanatos. # # |
Is PARP or Parthanatos involved with Parkinson’s?
This is not yet clear.
Researcher have reported increased levels of PARP in the dopamine neurons in the postmortem brain of people with Parkinson’s (Click here to read more about this). In addition, there have been reports suggesting that genetic mutations in the PARP genes may actually protect people from developing Parkinson’s (Click here and here to read more about this).
But what is apparent is that inhibiting PARP appears to be neuroprotective in models of neurological conditions.
For example, in 1998 this research report was published:
 Title: Inhibition of poly(ADP-ribose) polymerase: reduction of ischemic injury and attenuation of N-methyl-D-aspartate-induced neurotransmitter dysregulation.
Title: Inhibition of poly(ADP-ribose) polymerase: reduction of ischemic injury and attenuation of N-methyl-D-aspartate-induced neurotransmitter dysregulation.
Authors: Lo EH, Bosque-Hamilton P, Meng W.
Journal: Stroke. 1998 Apr;29(4):830-6.
PMID: 9550519 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers noticed that PARP levels increase dramatically in the brain after an ischemic injury (stroke), and they wondered whether blocking PARP would help or hinder the recovery. They modelled stroke in two groups of rats (one group was treated with the PARP inhibitor 3-AB and the other group were used as controls), and they analysed their brains 24 hours after the induction of stroke. The treated group were given the PARP inhibitor immediately after the stroke was induced.
The investigators found that treating the animals with the PARP inhibitor significantly reduced the area of damage in the brain (the reduction was over 50%).
And this finding got other researchers asking whether PARP could be involved in other neurological conditions, including Parkinson’s.
And that led to this report being published in 1999:
 Title: Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism.
Title: Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism.
Authors: Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM.
Journal: Proc Natl Acad Sci U S A. 1999 May 11;96(10):5774-5779.
PMID: 10318960 (This report is OPEN ACCESS if you would like to read it)
The researchers found that shorty after treating mice with a neurotoxin (MPTP, which kills dopamine neurons – one of the vulnerable populations of brain cells in Parkinson’s), the dopamine neurons in the brain would start to produce high levels of PARP.
In the image below, the dopamine neurons in the substantia nigra pars compacta (SNpc) – the region of the brain where dopamine neurons reside – are visible because the protein PARP has been stained red (after MPTP treatment):
 Source: PMC
Source: PMC
The researchers next genetically engineered some mice that do not produce PARP and they conducted an experiment in which the “no-PARP” mice and normal mice were treated with the MPTP neurotoxin. The investigators found that while the normal mice lost more than half of their dopamine neurons, the neurotoxin had little (if any) effect on the dopamine neurons in the no-PARP mice.
Other research groups have also replicated these results (Click here, here, here, here, and here to read some examples). Research groups have also found that inhibition of PARP reduces levels of the Parkinson’s associated protein alpha synuclein (Click here to read more about this).
In 2018, this research report was published:
 Title: Poly (ADP-ribose) drives pathologic alpha-synuclein neurodegeneration in Parkinson’s disease
Title: Poly (ADP-ribose) drives pathologic alpha-synuclein neurodegeneration in Parkinson’s disease
Authors: Kam TI, Mao X, Park H, Chou SC, Karuppagounder SS, Umanah GE, Yun SP, Brahmachari S, Panicker N, Chen R, Andrabi SA, Qi C, Poirier GG, Pletnikova O, Troncoso JC, Bekris LM, Leverenz JB, Pantelyat A, Ko HS, Rosenthal LS, Dawson TM, Dawson VL.
Journal: Science, 2018 Nov 2;362(6414). pii: eaat8407.
PMID: 30385548 (This report is OPEN ACCESS if you would like to read it)
In this study the researchers treated neurons grown in cell culture to preformed alpha synuclein fibrils.
What is alpha synclein fibrils?
Alpha synuclein is one of the most abundant proteins in our brains – making up about 1% of all the proteins floating around in each neuron in your head – and it is a very well studied protein (with over 10,000 research reports listed on the Pubmed search engine with the key words ‘alpha synuclein’).
When alpha synuclein protein is produced by a cell, it normally referred as an ‘unfolded protein’, in that is does not really have a defined structure. When it is first produced, alpha synuclein will look something like this:
Alpha synuclein. Source: Wikipedia
In this form, alpha synuclein is considered a monomer – which is a single molecule, just one copy of the protein. It is capable of binding to other molecules, and when it binds to other alpha synuclein proteins, they form what is called an oligomer (a collection of monomers). And these oligomers can have different structures.
In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of monomers, oligomers and fibrils. Source: Brain
And it is believed that these oligomer and fibril forms of alpha synuclein protein may go on to produce the Lewy bodies that characterise the Parkinsonian brain.
 Parkinson’s associated alpha synuclein. Source: Nature
Parkinson’s associated alpha synuclein. Source: Nature
By treating neurons grown in cell culture with preformed alpha synuclein fibrils, the researchers noted an increase in levels of both PARP and PAR, as well as an increase in cell death. When they treated these cells with PARP inhibitors, they found that the levels of PARP, PAR, and cell death all dropped dramatically.
Genetically removing PARP from the cells (using CRISPR gene editing technology) also protected neurons from the toxic effects of preformed alpha synuclein fibrils. These (and other experiments) suggested that preformed alpha synuclein fibrils were primarily killing neurons via parthanatos.
To confirm this result, the investigators next injected preformed alpha synuclein fibrils into both normal mice and mice that were genetically engineered to have no PARP.
And guess what?
Six months after being injected with preformed alpha synuclein fibrils, the dopamine neuron population in the normal mice was reduced by approximately 50%, but the mice with no PARP exhibited no dopamine cell loss. The researchers repeated this experiment with two groups of normal mice, but they treated one group with PARP inhibitor drugs. Again, after 6 months the PARP inhibitor treated mice exhibited little if any cell loss compared to their untreated counterparts (who suffered 50% dopamine neuron loss).
Now, because the rise in levels of PARP were associated with an increase in levels of PAR (when cells were treated with preformed alpha synuclein fibrils), the researchers asked what effect PAR itself may be having.
When they exposed normal human alpha synuclein protein to PAR in a solution, the investigators reported a marked acceleration of protein aggregation. In addition, when they looked at the brains of mice injected with preformed alpha synuclein fibrils, they found that 20% of alpha synuclein was bound to PAR.
On top of this result, exposing both normal cells and cells with no PARP to increased levels of PAR resulted in increased aggregation of alpha synuclein – which suggested to the researchers that it is PAR and not PARP that directly increases levels of alpha synuclein aggregation. By treating the cells with PARP inhibitors, the levels of PAR were also significantly reduced, which in turn resulted in less alpha synuclein aggregation.
This increase in PAR-induced alpha synuclein aggregation resulted in an raised levels of cell death in neurons grown in culture. And in the absence of alpha synuclein, they found that PAR was not toxic (even at very high levels).
Further investigations suggested that exposing preformed alpha synuclein fibrils to PAR was causing them to adopt an even more toxic state. And this demonstrated itself further when the researchers injected either PAR exposed preformed alpha synuclein fibrils or just normal preformed alpha synuclein fibrils into mice: the mice injected with PAR exposed preformed alpha synuclein fibrils had a more rapid loss of dopamine neurons and behavioural/motor problems.
The researchers summarised their report with the following conclusions:
- Alpha synuclein is killing cells by activating PARP (via the parthanatos cell death pathway)
- PARP activation leads to increased levels of PAR which accelerate alpha synuclein aggregation (a feed-forward cycle)
- PAR levels are increased in the brains of people with Parkinson’s
Given all of these results, the investigators suggested that clinically available PARP inhibitors should be considered for clinical testing in Parkinson’s
|
# # # RECAP #3: Inhibiting PARP appears to be neuroprotective in models of neurological conditions, including Parkinson’s. By blocking PARP, researchers have demonstrated the rescue of both neurotoxin and alpha synuclein models of Parkinson’s. # # # |
Interesting. Has a PARP inhibitor ever been tested in Parkinson’s?
Not that I am aware of (and I’m happy to be corrected on this).
PARP inhibitors are currently used in the treatment of certain cancers.
 PARP inhibition in cancer. Source: Parp-inhibitors
PARP inhibition in cancer. Source: Parp-inhibitors
The most common treatment for many cancers is chemotherapy, which functions by causing fatal DNA damage in cancer tumor cells. Key DNA repair pathways (like PARP), however, are hyperactive in a lot of cancers which results in increased resistance to chemotherapy treatment. But by inhibiting the DNA repair mechanism – by blocking PARP – researchers can potentiate the effect of chemotherapy, and increase the chances of killing off the tumor cells.
And this has resulted in the development of numerous PARP inhibitors by multiple pharmaceutical companies for clinical use in cancer.
Repurposing this class of drugs for Parkinson’s will be difficult, however, as most of the available PARP inhibitors have very poor brain penetration (by design). They all have a lot of trouble getting across the blood-brain-barrier – the protective membrane surrounding our brains. And simply using higher doses of these drugs to increase levels actually entering the brain is a non-starter, due to the side effects associated with the PARP inhibitors (side effects include nausea (generally in 50% of cases), fatigue (33%), anemia (low hemoglobin levels in the blood), vomiting, and neutropenia (low level of neutrophils, white blood cells important to fighting off infections)).
Most of the PARP inhibitors also bind to PARP when it is attached to the DNA, blocking its function. This mechanism of action can have a cytotoxic effect – killing the cell. Not an ideal drug for investigating neuroprotection in neurodegenerative conditions.
Click here to read a rather comprehensive review (written in early 2017) of repurposing PARP inhibitors for the therapy of non‐oncological conditions.
So no PARP inhibitors then?
So this is where we finally return to the report mentioned at the top of this post:
 Title: Activation oftheAkt1-CREB pathway promotes RNF146expression toinhibit PARP1-mediated neuronal death
Title: Activation oftheAkt1-CREB pathway promotes RNF146expression toinhibit PARP1-mediated neuronal death
Authors: Kim H, Park J, Kang H, Yun SP, Lee Y-S, Lee Y-I, LeeY
Journal: Science Signaling 22 Dec 2020, 13, 663, eaax7119.
PMID: N/A
In this study, the researchers were interested in a protein called PARP. In particular, they were interested in the ability of a protein called RNF146 to reduce PARP activity (that is, producing PAR).
How does RNF146 do that?
RNF146 appears to be able to recognise overactivate PARP and it regulates the activity by sending PARP off for waste disposal (via proteasomal degradation).
These researchers had previously published a report in 2017 which screened 640 natural compounds for their ability to activate RNF146. In this study, the researchers focused on liquiritigenin, which is an active estrogenic compound from the root of Glycyrrhizae uralensis. They found that administation of liquiritigenin activated RNF146 and that this resulted in reduced levels of PAR, which rescued a neurotoxin model of Parkinson’s.
This is the report of that study:
 Title: Estrogen receptor activation contributes to RNF146 expression and neuroprotection in Parkinson’s disease models.
Title: Estrogen receptor activation contributes to RNF146 expression and neuroprotection in Parkinson’s disease models.
Authors: Kim H, Ham S, Lee JY, Jo A, Lee GH, Lee YS, Cho M, Shin HM, Kim D, Pletnikova O, Troncoso JC, Shin JH, Lee YI, Lee Y.
Journal: Oncotarget. 2017 Oct 11;8(63):106721-106739.
PMID: 29290984 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers found that administration of liquiritigenin increased RNF146 levels in cells. As you can see in the image of the green stained cell below, the control solution (DMSO) does not increase levels of RNF146 (red dots) in the top row of images, as liquiritigenin in the bottom row of images:
 Source: PMC
Source: PMC
When they treated a mouse model of Parkinson’s (6-OHDA neurotoxin), the investigators found that liquiritigenin induced increases in RNF146 levels were able to protect the dopamine neurons (and this effect was associated with reducing levels of PAR):
 Source: PMC
Source: PMC
In this same 2017 study, the researchers also noticed that another agent in their drug screen – chlorogenic acid – exhibited the same properties (reducing PARP activity) and so they decided to investigate this in follow up research, which is the report we are reviewing today.
What is chlorogenic acid ?
Chlorogenic acid is a natural antioxidant compound found in coffee beans – it is one of the predominant classes of phenolic acids found in coffee. It is mainly used to lose weight and to lower blood pressure.
 Chlorogenic acid. Source: Wikipedia
Chlorogenic acid. Source: Wikipedia
The researchers were able to demonstrate that chlorogenic acid increases cell survival in cellular models of Parkinson’s (neurotoxin 6-OHDA induced). It does this by inhibiting PARP activation via increasing levels of RNF146 activity.
The researchers also reported that administration of chlorogenic acid in mice treated with the neurotoxin 6-OHDA could rescue the neurodegeneration associated with this model of Parkinson’s. Chlorogenic acid alone was not toxic to dopamine neurons, but rather increased levels of RNF146 in these cells. This result is supported by a previous study reporting that chlorogenic acid could rescue an MPTP mouse model of Parkinson’s (Click here to read that report).
And the investigators further assessed this result in another (more disease-relevant) model of Parkinson’s: the alpha-synuclein preformed fibril model – that we mentioned above. The researchers reported that administration of chlorogenic acid reduced the behavioural issues associated with this alpha synuclein model and protected the mice from dopamine neurodegeneration.
Interestingly, the scientists finished off their study by looking at postmortem brains from people who passed away with Parkinson’s and they found reduced levels of RNF146 in the PD brains (compared with controls), suggesting that insufficient levels of RNF146 could possibly be involved with the pathology of Parkinson’s.
Wow. Has chlorogenic acid ever been shown to inhibit PARP by other researchers?
Actually it has.
This report was published in 2015:
 Title: Targeting Human Poly(ADP-Ribose) Polymerase-1 with Natural Medicines and Its Potential Applications in Ovarian Cancer Therapeutics.
Title: Targeting Human Poly(ADP-Ribose) Polymerase-1 with Natural Medicines and Its Potential Applications in Ovarian Cancer Therapeutics.
Authors: Song M, Li JL, Li XP, Kan SF.
Journal: Arch Pharm (Weinheim). 2015 Nov;348(11):817-823.
PMID: 26344206
In this study, the investigators screened more than 130,000 commercially available natural products on ovarian cancer cells to identify novel potent PARP inhibitors.
Chlorogenic acid came in second place (with a IC50 of 25 nM).
Ok. And has chlorogenic acid ever been clinically tested in neurological conditions before?
Yes it has.
In 2019, this report was published:
 Title: Effect of Chlorogenic Acids on Cognitive Function in Mild Cognitive Impairment: A Randomized Controlled Crossover Trial.
Title: Effect of Chlorogenic Acids on Cognitive Function in Mild Cognitive Impairment: A Randomized Controlled Crossover Trial.
Authors: Ochiai R, Saitou K, Suzukamo C, Osaki N, Asada T.
Journal: J Alzheimers Dis. 2019;72(4):1209-1216.
PMID: 31683483 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers conducted a randomised, controlled, crossover clinical study evaluating chlorogenic acid in 34 individuals with mild cognitive impairment. Placebo or an active beverage containing chlorogenic acid (553.6 mg/bottle) was administered twice daily for 12 weeks. At the end of that treatment period, the investigators found that the chlorogenic acid treatment resulted in significantly fewer errors, which led them to conclude that “continuous intake of chlorogenic acid appears to improve attention and executive function among cognitive functions in mild cognitive impairment”. But they were also quick to state that it would be “important to conduct a long-term parallel trial to verify the effects of chlorogenic acid”.
As far as I’m aware, no clinical trial of chlorogenic acid has been conducted in Parkinson’s (and – again – I am happy to be corrected on this).
So what does it all mean?
There is ample epidemiological research indicating that coffee intake is associated with a reduced risk of developing Parkinson’s. The mechanism by which this neuroprotective effect may be occurring is unknown – there are a lot of chemicals in coffee. Recently, however, researchers in South Korea have highlighted one active component of coffee – chlorogenic acid – and an interesting mechanism of action (inhibition of PARP) that could help to explain the beneficial effect of coffee.
This new result is intriguing, but it now needs to be independently replicated and further assessed before we get too excited. We will also need to investigate potential biomarkers that could be used to assess target engagement and provide a potential read out on RNF146 and PARP activity before any clinical trials should be initiated.
It will be interesting to see if 2021 can give these things to us, but until then maybe sit back and have another cuppa.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Collins & Wikipedia


Great post. So keep drinking that strong real coffee and eating licorice! (Not together – urgh).
Is there any study into incidence, or more importantly rate of progression of PD in populations that do both? I was thinking of the Dutch, who seem to drink coffee all day long, and eat vast amounts of salty licorice / drop.
LikeLiked by 1 person