|
# # # # You may not realise it, but the DNA in your cells is under constant attack. All kinds of stressors (like the damaging effects of oxidative stress resulting from cellular processes) are constantly bombarding this precision molecule that contains the genetic blueprint for making and maintaining you. Luckily, millions of years of evolution has led to a complex and comprehensive DNA repair system that never takes holidays…. but might become a little slower as we age. Recently researchers have reported that certain aspects of this DNA repair system could be playing a role in Parkinson’s. In today’s post, we will review some new research in this area and consider the implications of the findings. # # # # |
My daughter is entering the pre-teen years, and I am struggling with all the horrors that that age brings.
Having survived the ‘Terrible Twos’ and the ‘Three-nager’ phase, I have absolutely adored innocence and magic of years 4 to 8. They were delightful. The ninth year, however, has brought with it the ominous arrival of (for lack of a better word) sass.
It has also involved a departure from the childhood songs (think Disney’s Lion King, Frozen, or Moana hits), and the introduction of more modern music, like her current favourite Meghan Trainor’s All about that Bass (see video above).
The next decade $£%#!& terrifies me.
But Meghan’s song provides an appropriate background for the subject matter of today’s post: Base excision repair
(Yeah, I know that’s a strange segway, but I’m tired and lacking imagination tonight)
What is Base excision repair?
Base excision repair is a process that corrects small base lesions that do not significantly distort the DNA helix structure.
What does any of that actually mean?
Let’s start at the beginning with the main subject matter: DNA
In almost every cell of your body you have DNA (or Deoxyribonucleic acid). It is a molecule that is composed of two chains which coil around each other, forming the famous double helix shape.
 The double helix. Source: Pngtree
The double helix. Source: Pngtree
The information stored in DNA provides the template or the instructions for making (and maintaining) a particular organism. All of the necessary details are encoded in that amazing molecule.
How is information stored in DNA?
In the image above, you can see strands reaching between the two chains of the DNA molecule – like the rungs on a ladder – and it is these numerous strands that contain the information.
These strands are called ‘nucleotides’.
These are organic molecules which contain one of four bases – the familiar A, C, T & Gs that make up our DNA code. These nucleotides form pairs (called ‘base pairs’) which then join together in long strings of DNA.
 The basics of genetics. Source: CompoundChem
The basics of genetics. Source: CompoundChem
It takes a lot of instructions to make a fully functional human being, and as a result human DNA contains a lot of these base pairs – over 3 billion of them. And having so many base pairs results in a lot of DNA in each cell – 2 meters of it. In order to get all that DNA crammed into the nucleus of a tiny little cell, DNA is wound up tightly into thread-like structures called chromosomes.
 DNA in a chromosome. Source: Byjus
DNA in a chromosome. Source: Byjus
Humans have 23 pairs of chromosomes, all jam packed with base pairs.
This video does a better job of explaining DNA than I do:
All along each string of DNA, there are regions that can be ‘read’ and copied (or transcribed) into RNA – this is the first step in the production of protein. Each of these regions is called a gene.
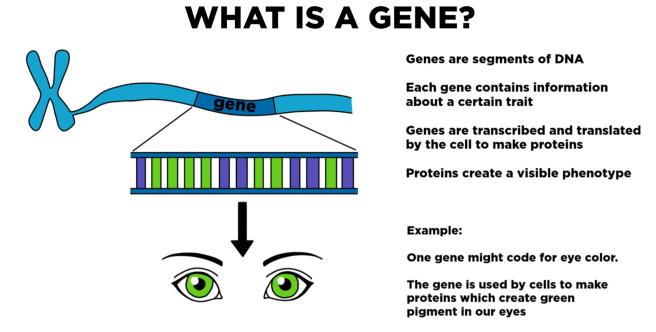 Source: Expii
Source: Expii
Genes can provide the instructions for making a particular protein. They are a sequence of base pairs that help to make our bodies function normally.
Ok, but what does base excision repair mean?
Base excision repair (or BER) is a cellular mechanism that helps to repair damaged DNA.
The base excision repair process is primarily responsible for dealing with small, non-distorting base damage to DNA – removing bases that have been lesioned and need to be replaced. There is also a related mechanism called the nucleotide excision repair pathway which handles more bulky DNA-distorting damage. As you can see in the image below, both processes involve a lot of necessary components:
 Source: Mdpi
Source: Mdpi
What can cause this damage? What does ROS mean in the image above?
Many things can cause damage to DNA, but one of the most common stressors in oxidative stress.
What is oxidative stress?
Oxidation is the loss of electrons from a molecule, which in turn destabilises that particular molecule.
Think of iron rusting. Rust is the oxidation of iron – in the presence of oxygen and water, iron molecules will lose electrons over time. Given enough time, this results in the complete break down of objects made of iron.
 Rusting iron. Source: Thoughtco
Rusting iron. Source: Thoughtco
The exact same process occurs in biology. Molecules in your body go through a similar process of oxidation – losing electrons and becoming unstable. This chemical reaction leads to the production of what we call free radicals. One type of free radical are reactive oxygen species (or ROS – you will remember your question regarding this acronym).
ROS is an umbrella term for an large array of derivatives of molecular oxygen that occur through the normal cellular functioning of cells. And because they produce so many ROS from their continual cellular processes, our cells have developed an assortment of defense systems against these little blighters.
One common defense is the production of antioxidants.
What is an antioxidant?
While free radicals are the bad guys in oxidation, antioxidants can be considered the good guys. They are molecules that neutralize the free radicals by donating one of their own electrons. The antioxidant do not become free radicals by donating an electron because by their very nature they are stable with or without that extra electron.
 How free radicals and antioxidants work. Source: h2miraclewater
How free radicals and antioxidants work. Source: h2miraclewater
Elevated levels of different ROS and free radicals leads to cellular damage, which is referred to as ‘oxidative stress‘, and this can have really serious consequences, resulting in damage to DNA and ultimately leading to the death of the cell.
 Source: News-medical
Source: News-medical
And it is ROS and oxidative stress that can cause small, non-distorting base damage to DNA that requires the activation of the base excision repair process.
As we age, there is an increase in levels of oxidative stress in our bodies – sorry to say this, but it is a battle that we are all losing as we grow older (like my Dad says “Aging ain’t for sissies“).
Given our tendency to age and the elevation in levels of oxidative stress as we get older, researchers have been curious about how our DNA repair mechanisms (like BER) adapt over time, and if they might be involved with progressive conditions like Parkinson’s.
|
# RECAP #1: DNA is a molecule that is critical to life, but extremely vulnerable to damage (via processes like oxidative stress). Various mechanisms of DNA repair have evolved which help to keep cells health. The base excision repair process is one of these repair mechanisms – removing and replacing damaged bases in the long chain of the DNA thread. # |
Ok, so oxidative stress can cause damage to DNA and the base excision repair process is involved in repairing that damage. Got it. But how does any of this relate to Parkinson’s?
Well, previous research has demonstrated that DNA from sections of postmortem midbrain collected from people who passed away with Parkinson’s had significantly higher number of strand breaks (compared to age-matched control brains – click here to read more about this).
And this has been validated by other research groups (Click here and here to read more examples).
This research suggests that elevated levels of oxidative stress are possibly playing a role in Parkinson’s, or that reductions in the activity of DNA repair processes could be involved.
And very recently, a report was published that presented evidence suggesting that the latter of these two possibilities could be at play.
Here is the report in question:
 Title: Base excision repair causes age-dependent accumulation of single-stranded DNA breaks that contribute to Parkinson disease pathology.
Title: Base excision repair causes age-dependent accumulation of single-stranded DNA breaks that contribute to Parkinson disease pathology.
Authors: SenGupta T, Palikaras K, Esbensen YQ, Konstantinidis G, Galindo FJN, Achanta K, Kassahun H, Stavgiannoudaki I, Bohr VA, Akbari M, Gaare J, Tzoulis C, Tavernarakis N, Nilsen H.
Journal: Cell Rep. 2021 Sep 7;36(10):109668.
PMID: 34496255 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers were interested in determining the effect of incomplete base excision repair in aging. To achieve incomplete base excision repair, they focused their attention on Endonuclease III-like protein 1.
What is Endonuclease III-like protein 1?
Endonuclease III-like protein 1 is an enzyme that in humans is encoded by the NTHL1 gene (and for the rest of this post, to keep things simple, we will refer to Endonuclease III-like protein 1 as simply “NTH-1”).
NTH-1 is a bifunctional DNA glycosylase whose job it is to dig damaged bases out of strands of DNA.
What is a DNA glycosylase?
DNA glycosylases are a family of enzymes that initiate the first step of the base excision repair process. They identify and remove the damaged base from the strand of DNA while leaving everything else intact.
In the image below, a section of DNA has been damaged by oxidative stress and a base (in black) needs to be removed by the DNA glycosylase NTH-1:
 Source: Cell
Source: Cell
OK, so NTH-1 comes in and then detects and removes damaged bases in DNA. Got it. What did the researchers do next?
They engineered a C. elegans model of Parkinson’s.
What is C. elegans?
Caenorhabditis elegans (or simply C. elegans) are transparent nematode – also known as roundworms. They are about 1 mm in length, and they have an extremely well characterised nervous systems (C. elegans have 302 neurons and 56 glial cells in total, which communicate through approximately 6400 chemical synapses, 900 gap junctions, and 1500 neuromuscular junctions – like I said, they are extremely well characterised!).
 Caenorhabditis elegans – cute huh? Source: Nematode
Caenorhabditis elegans – cute huh? Source: Nematode
Given their thoroughly mapped out nervous systems, C. elegans provide a useful tool for studying biology. They are easy to grow/maintain, they have an overall life span of 2-3 weeks, and researchers have developed a wide range of tools that allow for genetic manipulation to address specific questions.
The researchers bred C. elegans that produced very high levels of the Parkinson’s-associated protein alpha synuclein in their dopamine neurons. These C. elegans experienced a progressive loss of their dopamine neurons as they aged. Next the investigators repeated the experiment, but with a second group of C. elegans that produced very high levels of alpha synuclein, but had been genetically engineered to not produce any NTH-1 (NTH-1-deficient). Curiously, these C. elegans did not exhibit a progressive loss of their dopamine neurons as they aged.
These and other results suggested that NTH-1 deficiency actually protected the C. elegans against age- and oxidative-stress-related degeneration.
Isn’t this the opposite of what they might have expected?
Yep. Biology is funny like that.
They also found that NTH-1 deficiency improved dopamine-dependent behavior in the C. elegans.
Wait. What? This doesn’t make sense.
I know. One might expect that messing with DNA repair would result in cells shutting down functions and ultimately dying, but instead these cells… improved.
So what did the researchers make of this result?
They conducted more experiments and found that it came down to mitohormesis.
And what is mitohormesis?
I have to admit that this was a new word for me too.
Mitohormesis is a biological response in which a small amount of mitochondrial stress leads to an increase in health and viability within a cell, tissue, or organism. Remarkably, a mild increase in the level of mitochondrial stress appears to improve mitochondrial function and leave cells less susceptible to future perturbations. But there is a limit to this – the effect has ‘Goldilocks-like’ nature to it – and more severe levels of cellular stress will lead to reduced mitochondrial function and cell death:
 Source: PMC
Source: PMC
For those interested in learning more about (Click here and here to read reviews on the topic).
But how did they make the mitohormesis connection?
The researchers noted that NTH-1 is actively working on DNA in the nucleus,… but also in mitochondria, and this got them thinking.
Remind me again: What are mitochodria?
Mitochondria are the power house of each cell. They provide the cell with energy, which helps to keep the lights on. Without them, the lights go out and the cell dies.
A mitochondrion (singular) and its location in the cell. Source: NCBI
An interesting detail to note regarding Parkinson’s and mitochondria is that many of the early onset forms of Parkinson’s are associated with genetic variations in genes that are involved with normal mitochondrial functioning (such as PINK1 & PARKIN – Click here and here to read previous SoPD posts about this).
The researchers knew that in the absence of NTH-1, C. elegans (non-PD model) displayed mild mitochondrial dysfunction, which has been previously reported (Click here to read more about this). This dysfunction resulted in a chronic increase in levels of ROS, which in turn leads to an increase in antioxidant-associated responses. And it was this increase that explained the neuroprotection via mitohormesis.
|
# # RECAP #2: Researchers found that a deficiency in one component of the DNA repair system, results in a neuroprotective effect, rather than an enhanced level of cell death. This effect was due to mitohormesis – a neuroprotective effect resulting from a mild increase in mitochondrial stress eliciting a boost in antioxidant activity within cells. # # |
Interesting. And they think this occurs in Parkinson’s?
Well, next they looked at two independent Parkinson’s cohorts to assess whether base excision repair might influence the risk of developing Parkinson’s in humans. The cohorts used were the the Norwegian ParkWest cohort and the Parkinson’s Progression Markers Initiative. By analysing the genetic data of these two pools of data, the researchers found a significant enrichment of genetic variants in genes associated with base excision repair, particularly a DNA glycosylase called NEIL2. One particular variant was detected in 6 of 411 PD cases and 0 of 640 controls.
The researchers concluded that the identified genetic variants “may contribute to susceptibility of PD in humans“, but are likely to have only “a modest effect on PD risk“.
Interesting. So, summing up? What does it all mean?
Before we do that there was several other interesting aspects to this study.
First, during their analysis the researchers assessed a PARP inhibitor in their C. elegans that produced high levels of alpha synuclein.
What is PARP?
Poly (ADP-ribose) polymerase (or PARP) is a family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death.
The main function of PARP in cells is to detect DNA damage and initiate a response.
How does it do that?
When damaged DNA is detected, PARP will bind to the DNA and begin to synthesize chains of poly-ADP ribose (or PAR). These tendrils of PAR serve as a signaling mechanism for DNA-repairing enzymes, making them aware of the damage and recruiting their help to fix it.
 PARP initiating DNA repair mechanisms. Source: bpsbioscience
PARP initiating DNA repair mechanisms. Source: bpsbioscience
This is a very good system if the DNA damage is not too bad and if PARP activity is kept under strict control.
PARP inhibition has previously been shown to be neuroprotective in models of Parkinson’s. We have discussed this in previous SoPD posts on PARP (Click here and here to read those posts).
In the report reviewed today, the investigators also found that PARP inhibition was neuroprotective in their C. elegans that produced high levels of alpha synuclein.
Mmm, interesting. And what was the second interesting aspects to this study?
The researchers also demonstrated that nicotinamide riboside was neuroprotective in their C. elegans that produced high levels of alpha synuclein.
What is nicotinamide riboside?
It is one of three forms to vitamin B3. The three are:
- Nicotinic acid (NA or Niacin)
- Nicotinamide (Nam)
- Nicotinamide riboside (NR)
The different forms of Vitamin B3. Source: Longecity
All three forms are used by your body in the production of nicotinamide adenine dinucleotide.
What is nicotinamide adenine dinucleotide?
Nicotinamide adenine dinucleotide (or NAD) is a protein that plays a very critical role in a wide range of cellular reactions. Importantly, it is a required co-factor in a process of passing hydrogen electrons from one protein to another, which is essential for the continued production of energy (in the form of ATP) by the mitochondria in cells.
The mitochondria convert nutrients from food into Adenosine Triphosphate (or ATP). ATP is the fuel which cells run on. Given their critical role in energy supply, mitochondria are plentiful (some cells have thousands) and highly organised within the cell, being moved around to wherever they are needed.
Source: Mangomannutrition
Without NAD, the production of ATP starts to go wrong very quickly. NAD is present in every cell and it is essential for normal functioning. If this process of NAD production is of interest to you, click here for a very good review of the topic.
It is interesting that the researchers behind today’s report found that nicotinamide riboside was neuroprotective in their C. elegans that produced high levels of alpha synuclein.
Interesting yes, but these experiments were performed in microscopic worms. Have PARP inhibitors or nicotinamide riboside been clinically tested in Parkinson’s?
PARP inhibition has not.
But nicotinamide riboside is currently being tested in the large NOPARK study in Norway.
 Norway. Source: Go-today
Norway. Source: Go-today
The NOPARK study is a Phase II multi-center, double-blinded, randomised trial of nicotamide riboside in 400 people with recently diagnosed Parkinson’s. The participants are being treated with either nicotamide riboside (500 mg x 2/day) or placebo for one year. The results of the study are expected in 2024 (Click here to read more about this).
The investigators in Norway have also recently completed a small pilot trial called the NAD-PARK study. It was a double-blinded randomised investigation of nicotamide riboside in 30 drug naïve people with Parkinson’s. They were given either nicotamide riboside (500 mg x 2/day) or placebo for 4 weeks and brain imaging was used to assess the outcome of the study (Click here to read more about this).
We look forward to seeing this pilot study data in the not-too-distant future.
So what does it all mean?
Neurodegenerative conditions are primarily associated with aging. Given an increase in levels of oxidative stress over time in our bodies, researchers have naturally been curious about the impact that this process could be having on the maintenance and repair mechanisms of DNA.
Recently scientists have found that deficiency in a particular component of DNA repair – base excision repair – has a surprising effect on the function of dopamine neurons in C’ elegans: It protects them and makes them function better, via a process known as mitohormesis (incremental increase in mitochondrial stress results in an elevation in cellular antioxidant processes which is protective).
The basic biological effect is an interesting result, but the researchers also provided further data supporting PARP inhibition and NAD supplementation in their models of Parkinson’s. Clinical trials evaluating NAD elevation are being conducted. Further investigations into oxidative stress, DNA integrity and Parkinson’s will hopefully provide additional insights and potential avenues for therapeutic interventions.
Oh yeah, and my daughter also loves listening to “Despacito” with the volume turned up load (again, not a natural segway. Just call it a desperate cry for help!):
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from Pinterest.




‘Yep. Biology is funny like that’
Indeed!
~
Many in longevity community say not to take NAM as NAD precursor since it is a PARP inhibitor. There’s evidence NR just gets converted to NAM by liver, but C. elegans don’t have livers. Will have to read the paper & also your previous posts on PARP.
NAM is a lot cheaper than NR.
LikeLike