|
# # # # This week the biotech firm Denali Therapeutics made two major announcements regarding the development of their LRRK2 inhibitor program for Parkinson’s. First, the company revealed that they have signed an agreement with the pharmaceutical company Biogen to co-develop and co-commercialise small molecule inhibitors of LRRK2 for Parkinson’s. Second, Denali also announced that they have a green light from the US FDA to start the next phase of clinical testing of their LRRK2 inhibitor DNL151. In today’s post, we will discuss what is meant by LRRK2 inhibitor, what the details of the announcements are, and what all of this means for the Parkinson’s community. # # # # |
 Denali. Source: Wikipedia
Denali. Source: Wikipedia
Peaking at 20,310 feet (or 6,190 m) above sea level, Denali (Koyukon for “the high one”; also known as Mount McKinley) is the highest mountain in North America. The first verified ascent of this Alaskan mountain occurred on June 7, 1913, when four climbers (Hudson Stuck, Harry Karstens, Walter Harper, and Robert Tatum) conquered it.
 Tatum (left), Karstens (middle), and Harper (right). Source: Gutenberg
Tatum (left), Karstens (middle), and Harper (right). Source: Gutenberg
Robert Tatum later commented, “The view from the top of Mount McKinley is like looking out the windows of Heaven!”
More recently another adventurous group associated with ‘Denali’ have been trying to scale lofty heights, but of a completely different sort to the mountaineering kind.
 Founded in 2013 by a group of former Genentech executives, San Francisco-based Denali Therapeutics is a biotech company which is focused on developing novel therapies for people suffering from neurodegenerative diseases. Although they have product development programs for other condition (such as Amyotrophic Lateral Sclerosis and Alzheimer’s disease), Parkinson’s is definitely their primary indication of interest.
Founded in 2013 by a group of former Genentech executives, San Francisco-based Denali Therapeutics is a biotech company which is focused on developing novel therapies for people suffering from neurodegenerative diseases. Although they have product development programs for other condition (such as Amyotrophic Lateral Sclerosis and Alzheimer’s disease), Parkinson’s is definitely their primary indication of interest.
And this week, the company made two major announcements with regards to their Parkinson’s research program.
The first announcement was that Denali have signed an agreement with the pharmaceutical company Biogen to co-develop and co-commercialise small molecule inhibitors of LRRK2 for Parkinson’s (Click here to read the press release).
Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’) – also known as ‘Dardarin‘ (from the Basque word “dardara” which means “trembling”) – is an enzyme that has many functions within a cell – from supporting efforts to move things around inside the cell to helping to keep the power on (involved with mitochondrial function).

The many jobs of LRRK2. Source: Researchgate
The LRRK2 gene is made up of many different regions. Each of those regions is involved with the different functions of the eventual protein. As you can see in the image below, the regions of the LRRK2 gene have a variety of different functions:
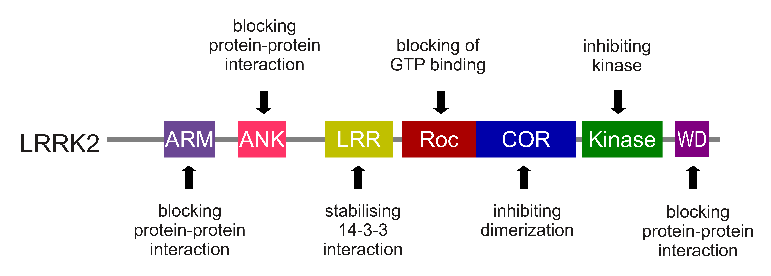
The regions and associated functions of the LRRK2 gene. Source: Intechopen
Genetic errors (or ‘variations’) within the LRRK2 gene are recognised as being some of the most common with regards to increasing ones risk of developing Parkinson’s (LRRK2 variants are present in approximately 1-2% of all cases of Parkinson’s).

The structure of Lrrk2 and where various mutations lie. Source: Intech
As the image above suggests, variations in the PARK8 gene are also associated with Crohn’s disease (Click here and here for more on this) – though that variation is in a different location to those associated with Parkinson’s. And one particularly common Parkinson’s-associated LRRK2 variation – called G2019S – is also associated with increased risk of certain types of cancer, especially for hormone-related cancer and breast cancer in women – Click here to read more about this. If you have a G2019S variation, there is no reason to panic – but it is good to be aware of this association and have regular check ups.
The G2019S variation (the name designates its location on the gene) is the most common LRRK2 mutations. In some populations of people it can be found in 40% of people with Parkinson’s (Click here to read more about this).
Why do Biogen and Denali want to inhibit this protein?
Because the G2019S variation does not make the LRRK2 protein inactive, like we see with other mutant proteins. Rather, this variation results in a hyperactive form of LRRK2.
 LRRK2 protein. Source: Youtube
LRRK2 protein. Source: Youtube
Now, as a protein, LRRK2 interacts with many different types of other proteins, and you can imagine that in a finely balanced environment like the cells that a mutant ‘hyperactive’ form of LRRK2 is going to cause problems. The consequences of this constantly active form of LRRK2 protein is believed to be influential in the cell death in LRRK2-associated Parkinson’s.
Thus, LRRK2 inhibitors are being developed by biotech firms to try and tone down the hyperactive LRRK2 protein – hopefully slowing the progression of Parkinson’s.
Denali Therapeutics has been leading the charge in this area of research – they have two LRRK2 inhibitors called DNL-201 and DNL-151 in clinical testing for Parkinson’s – and reecently Biogen has decided that they want to hep this effort.
|
# RECAP #1: LRRK2 is a Parkinson’s-associated gene (functional region of DNA). The LRRK2 gene provides instructions for producing a multi-functional protein. When mutated, the gene provides instructions for a hyperactive form of the LRRK2 protein, which is believed to disrupt normal cellular function. Multiple biotech companies are developing therapies that inhibit the hyperactive form of the LRRK2 protein. # |
What are the details of the agreement between the companies?
Under the agreement, Biogen and Denali will co-develop and co-commercialise Denali’s small molecule inhibitors of LRRK2 for Parkinson’s. To partner up, Biogen is making an upfront payment of US$560 million to Denali Therapeutics, and they are also taking a US$465 million equity position in the company (13.3 million newly issued shares, representing 11.2% of the total shares). In addition, there are potential milestone payments (up to US$1.125 billion), profit sharing and royalties are part of the deal.
Biogen and Denali will share responsibility and costs for global development (60% Biogen; 40% Denali), as well as profits and losses for commercialisation in the U.S. (50% Biogen; 50% Denali) and China (60% Biogen; 40% Denali). Outside of the U.S. and China, Biogen will be responsible for commercialisation and (if all goes well) pay Denali tiered royalties.
On top of all of this, Biogen gets exclusive option rights to 2 programs for neurodegenerative diseases that utilise Denali’s new TV technology platform (including for Alzheimer’s-related beta amyloid), and right of first negotiation for 2 additional unnamed TV platform programs.
What are TV platform programs?
The “Transport Vehicle” (TV) technology platform in a new development from Denali that could greatly enhance our ability to treat neurological conditions. The technology was highlighted in two research reports published in May.
This is the first report:
 Title: Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys.
Title: Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys.
Authors: Kariolis MS, Wells RC, Getz JA, Kwan W, Mahon CS, Tong R, Kim DJ, Srivastava A, Bedard C, Henne KR, Giese T, Assimon VA, Chen X, Zhang Y, Solanoy H, Jenkins K, Sanchez PE, Kane L, Miyamoto T, Chew KS, Pizzo ME, Liang N, Calvert MEK, DeVos SL, Baskaran S, Hall S, Sweeney ZK, Thorne RG, Watts RJ, Dennis MS, Silverman AP, Zuchero YJY.
Journal: Sci Transl Med. 2020 May 27;12(545):eaay1359.
PMID: 32461332
In this study, the researchers were interested improving the ability of molecules to cross the blood-brain-barrier and access the central nervous system.
What is the blood-brain-barrier?
The blood-brain barrier is a protective membrane that surrounds the brain, separating circulating blood from the cerebrospinal fluid that the brain sits in. It is made up of endothelial cells that are connected by tight junctions. These cells limit the ability of many molecules (particularly large ones) to access the brain, making it a ‘barrier’ for many medications.
 The blood-brain-barrier. Source: Bioninja
The blood-brain-barrier. Source: Bioninja
The Denali researchers employed an antibody approach to try and deal with this issue.
What is an antibody?
Antibodies are Y-shaped proteins that the immune system naturally and continuously produces to identify anything in the body that is ‘not self’ (that is, not a normally occurring part of you – think of viruses, bacteria, etc).

Monoclonal antibodies. Source: Astrazeneca
Antibodies act like alert flags for the immune system. When antibodies bind to something, they alert the immune system to investigate and potentially remove. But the true beauty of each antibody is that it targets a very specific structure, while ignoring everything else. In this manner, antibodies can designed by researchers for use in targeting specific proteins.
The Denali researchers designed an antibody that would target transferrin receptor.
What is transferrin receptor?
The delivery of iron across the blood-brain-barrier to the brain is absolutely critical for normal neurological function, and it is accomplished via the binding of iron to a protein called transferrin.
The transferrin receptor is a protein that sits on the surface of endothelial cells that make up the blood-brain-barrier, and it is responsible for transporting the transferrin protein loaded with iron across the barrier and into the brain.
 Entering the brain. Source: Researchgate
Entering the brain. Source: Researchgate
Now the Denali scientists designed an antibody that would bind to the transferrin receptor, and is then trasferred into the brain (antibodies generally do not access the brain very well). But they designed their antibody so that it does not have the Y-shape of a classical antibody (left image below). Instead they removed the branches of the “Y”, leaving just the targetting mechanism of the antibody (which has the blood-brain-barrier (BBB) target epitope – middel image below). This allows them to attach any protein to this antibody (or “transport vehicle”), which in can then be transferred into the brain (right image below):
 Adapted from Alzforum (which has a good write up about this research)
Adapted from Alzforum (which has a good write up about this research)
As a proof-of-principle testof this transport vehicle, the Denali investigators selected an antibody for the Alzheimer’s-associated protein β-secretase 1 (BACE1). When they bound the BACE1 antibody to the transport vehicle and injected it into mice and primates, it resulted in substantially improved delivery to the brain (40-fold higher than BACE1 antibody alone) and a sustained pharmacological response (reduced soluble brain β-amyloid levels 57% more than BACE1 antibody alone).
This tecnology has many researchers very excited, as it has the potential to “create major changes in our ability to deliver therapies into the brain” (Source). There are some questions regarding the integrity of the blood-brain-barrier in neurodegenerative conditions, like Alzheimer’s and Parkinson’s, but the TV technology could make it possible to deliver a lot of drugs that were previously blocked from entering the brain.
And the Denali researchers demonstrated this in a second report that was published in parallel to the first:
 Title: Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice.
Title: Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice.
Authors: Ullman JC, Arguello A, Getz JA, Bhalla A, Mahon CS, Wang J, Giese T, Bedard C, Kim DJ, Blumenfeld JR, Liang N, Ravi R, Nugent AA, Davis SS, Ha C, Duque J, Tran HL, Wells RC, Lianoglou S, Daryani VM, Kwan W, Solanoy H, Nguyen H, Earr T, Dugas JC, Tuck MD, Harvey JL, Reyzer ML, Caprioli RM, Hall S, Poda S, Sanchez PE, Dennis MS, Gunasekaran K, Srivastava A, Sandmann T, Henne KR, Thorne RG, Di Paolo G, Astarita G, Diaz D, Silverman AP, Watts RJ, Sweeney ZK, Kariolis MS, Henry AG.
Journal: Sci Transl Med. 2020 May 27;12(545):eaay1163.
PMID: 32461331
In this study, the researchers used the TV technology to rescue a model of Hunter syndrome.
What is Hunter syndrome?
Hunter syndrome (or mucopolysaccharidosis type II) is a very rare, inherited genetic disorder that is caused by a missing or malfunctioning enzyme (called iduronate 2-sulfatase or IDS). IDS is required to break down large sugar molecules called glycosaminoglycans (or GAGs) in cells. In Hunter syndrome, GAGs accumulate and cause trouble.
The existing IDS enzyme replacement therapy (called idursulfase) is unable to access the brain due to the blood-brain-barrier, so the Denali researchers constructed a TV tethered to a molecule of IDS (ETV:IDS) and used it to correct both in vitro and in vivo models of Hunter syndrome.
The researchers concluded that their study demonstrated not only the therapeutic potential of the TV technology, but also provided “preclinical proof of concept for TV-enabled therapeutics to treat CNS diseases more broadly “.
If the TV technology works in clinical testing, this could seriously be a game changer for treating neurological conditions.
So you can see why Biogen is prepared to place a billion dollar bet.
Has the TV technology been tested in clinical trials yet?
Not yet, but Denali submitted an Investigational New Drug (or IND) application to the US FDA in late December 2019 for an agent called DNL310 (or ETV:IDS), which will be intravenously administered into individuals with Hunter syndrome (Click here to read the Denali press release discussing this).
|
# # RECAP #2: Denali and Biogen have announced a deal in which they will co-develop and co-commercialise Denali’s small molecule inhibitors of LRRK2 for Parkinson’s. In addition, Biogen will have exclusive option rights to two programs for neurodegenerative diseases that utilise Denali’s new “Transport vehicle” technology platform, which may allow for the delivery of therapeutic molecules that were previously blocked from accessing the brain. # # |
Ok, so are other companies developing LRRK2 inhibitors as well?
Yes, this area of research is fast becoming rather crowded and very busy.
One pharmaceutical company with a major LRRK2 inhibitor research program is GlaxoSmithKline. They appear to be gearing up for clinical trials, as they have paired up with genotyping company 23andMe to help identify individuals with LRRK2 genetic variants (Click here to read more about this).
 Other biotech companies building a LRRK2 inhibitor program include Cerevel Therapeutics.
Other biotech companies building a LRRK2 inhibitor program include Cerevel Therapeutics.
 This is a biotech firm that was started by Bain Capital and the Pharmaceutical company Pfizer (Click here to read more about this). Cerevel has taken on many of the neuroscience treatments that Pfizer was clinically testing until it shut down their neuroscience division in early 2018. In addition to those clinically tested assets, Cerevel have also quietly added ‘LRRK2 inhibitor’ to their preclinical ‘lead development’ area of research (Click here to read more about this).
This is a biotech firm that was started by Bain Capital and the Pharmaceutical company Pfizer (Click here to read more about this). Cerevel has taken on many of the neuroscience treatments that Pfizer was clinically testing until it shut down their neuroscience division in early 2018. In addition to those clinically tested assets, Cerevel have also quietly added ‘LRRK2 inhibitor’ to their preclinical ‘lead development’ area of research (Click here to read more about this).
A biotech company called E-Scape Bio is also developing a LRRK2 inhibitor program (Click here to read more about this):
 And there is a collaboration between Servier and Oncodesign to develop novel LRRK2 inhibitors (Click here to read more about this).
And there is a collaboration between Servier and Oncodesign to develop novel LRRK2 inhibitors (Click here to read more about this).
 The new deal between Biogen and Denali does raise questions, however, regarding another collaboration focused on LRRK2 inhibition.
The new deal between Biogen and Denali does raise questions, however, regarding another collaboration focused on LRRK2 inhibition.
The biotech company Ionis Pharmaceuticals is developing a novel LRRK2 approach. This company is conducting a Phase I clinical trial to evaluate their lead candidate, called BIIB094.
This company is conducting a Phase I clinical trial to evaluate their lead candidate, called BIIB094.
BIIB094 is an antisense oligonucleotide – this is an approach that blocks LRRK2 RNA before it can be used to make LRRK2 protein (we have discussed antisense oligonucleotides in a previous SoPD post – click here to read that post). The trial is a Phase I clinical trial to evaluate the safety and tolerability of single and multiple doses of BIIB094 (Click here to learn more about that trial). But Ionis is collaborating with a major pharmaceutical company in the development of BIIB094.
That pharma company is:
 Biogen appears to be doubling down on LRRK2 and placing some major bets that reducing the activity of this protein is the path forward for treating Parkinson’s.
Biogen appears to be doubling down on LRRK2 and placing some major bets that reducing the activity of this protein is the path forward for treating Parkinson’s.
|
# # # RECAP #3: The Denali/Biogen LRRK2 program is not working in isolation. There are numerous biotech companies developing LRRK2 inhibitors. There are also antisense oligonucleotide approaches being developed for Parkinson’s. # # # |
Interesting. You said there were two announcements from Denali. What was the second announcement that Denali made?
Yes, indeed.
There was a second announcement and it has more immediate implications for the Parkinson’s community. The same day as the collaboration with Biogen was announced, Denali also put out a press release regarding the next step in the clinical trial development of LRRK2 inhibitors (Click here to read the press release).
The company said that ““Safety and biomarker data from studies of our two LRRK2 molecules in Parkinson’s patients support moving DNL151 into late stage clinical studies with the aim of addressing the devastating clinical decline and pathology of disease in Parkinson’s“.
Both of the LRRK2 inhibitors that Denali has been developing – DNL201 and DNL151 – have demonstrated interesting results in Phase I clinical trials, but Denali has decided to select DNL151 for further clinical development due to “pharmacokinetic properties that provide additional dosing regimen flexibility” (meaning that it is the slightly better drug).
DNL151 has been tested in 162 healthy volunteers and 25 Parkinson’s patients in a Phase I testing, and Denali is currently completing a further dose escalation study in an expanded Phase I trial to better define the full therapeutic window of the molecule.
In July of this year, the US FDA cleared an Investigational New Drug (IND) application for DNL151 enabling expansion of the clinical trial program.
Denali and Biogen are now working on finalising plans for two separate Parkinson’s studies:
- One will be in patients with a LRRK2 genetic variant that causes hyperactivity of the protein, and
- The second study will involve participants with sporadic Parkinson’s (not associated with any genetic variant).
There are few details at present as to what these trials will look like, but Denali has previously been planning “Phase 2/3 clinical trials” for their LRRK2 inhibitors (Source), so we will hopefully be seeing a large Phase III trial or combination trial that will seamlessly shift from Phase II into a Phase III study.
Enrollment for these trials is expected to commence in 2021. Denali has set up a website (EngageParkinson’s) for anyone seeking to learn more.
So what does it all mean?
When everyone is bemoaning the departure of large pharmaceutical companies for the field of neurodegeneration (Click here to read an old SoPD post on this topic), it is encouraging to see a company like Biogen prepared to make a major investment in the development of novel disease modifying therapies for Parkinson’s. This appears to be part of a much broader neuroscience strategy for Biogen, who are currently attempting to get their immunotherapy candidate Aducanumab approved for Alzheimer’s (Click here to read more about this).
The announcement of the Denali/Biogen partnership is an exciting development for the Parkinson’s community as it not only brings news of the next stage in the clinical testing of Denali’s LRRK2 inhibitor program, but also brings a powerful new technology (the Transfer vehicle platform) closer to the clinic for neurodegenerative conditions. It will be particularly interesting to see which molecules are proposed for Parkinson’s using the TV platform. I suspect that there will be a lot of preclinical activity in this area over the next 12-18 months.
Meanwhile, we’ll be on the lookout for any new details regarding the new DNL151 LRRK2 inhibitor clinical trial.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, some of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
Further, the author of this post is an employee of the Cure Parkinson’s Trust. The Trust has not asked for this post to be written, and there has been no effort to highlight the work of the Trust over others (perceptions of any bias should be directed to the author). This post has been written by the author solely for the purpose of sharing what the author considers interesting information.
The banner for today’s post was sourced from the SoPD.

9 thoughts on “Billion dollar bets: Denali+Biogen”