|
Recent regulator approvals and exciting new preclinical data has refocused attention on a treatment approach for genetic conditions that has travelled a long and winding road towards clinical use. Antisense oligonucleotides represent a method of altering protein levels at the post transcriptional level – it basically stops certain RNAs from being translated into protein. And recently, a new clinical trial has been registered which will explore the use of this treatment approach in people with Parkinson’s. In today’s post, we will look at what antisense oligonucleotides are, how they work, what research has been conducted in the context of Parkinson’s, and some of the limitations of this approach that still exist.
|
 Source: Youtube
Source: Youtube
Spinal muscular atrophy (or SMA) is a genetic disorder that results in the degeneration of motor neurons in the spinal cord. This leads to progressive weakening and atrophy of muscules, ultimately leaving sufferers paralysed. It is caused by loss-of-function mutations in the survival motor neuron 1 (SMN1) gene.
It is a terrible condition that starts in very young children and has an incidence approaching 1:10,000 live births.
Luckily, novel therapies are being developed to deal with this condition, and in 2016, the US FDA approved a new treatment – following rather dramatic clinical trial results – called Nusinersen. This new therapy has caused a great deal of excitement as it basically halted the progression of SMA in many cases.
And a recent long term report highlights some of these very impressive results:
 Title: Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies.
Title: Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies.
Authors: Darras BT, Chiriboga CA, Iannaccone ST, Swoboda KJ, Montes J, Mignon L, Xia S, Bennett CF, Bishop KM, Shefner JM, Green AM, Sun P, Bhan I, Gheuens S, Schneider E, Farwell W, De Vivo DC; ISIS-396443-CS2/ISIS-396443-CS12 Study Groups.
Journal: Neurology. 2019 May 21;92(21):e2492-e2506.
PMID: 31019106 (This report is OPEN ACCESS if you would like to read it)
Most importantly, Nusinersen is having real impact on the children who are affected by this condition:
Interesting, but what exactly is Nusinersen?
It is an antisense oligonucleotide.
What are antisense oligonucleotides?
The golden rule in biology is DNA begets RNA, and RNA begets protein.
DNA provides the instructions for making a protein, and RNA is the readout of those plans which a cell can then translate into protein. DNA is the hard copy blueprint, while RNA is the print off which gets used and disposed of.
In medicine, when we need to reduce the levels of a particular protein in the body, we usually develop drugs that target a section of that protein and block its function. It is a system has served us well over the years, but over the last few decades researchers have been focusing more and more attention on inhibiting RNA rather than proteins.
Antisense oligonucleotides are one such method.
They are small pieces of DNA or RNA that are designed to target and bind to very specific strands of RNA. By doing this, the antisense oligonucleotide blocks the ability of that piece of RNA to be used to make a protein. This reduces the amount of that protein in the cell and the inhibited piece of RNA is eventually disposed of.
 Source: Conversation
Source: Conversation
Each antisense oligonucleotide is designed by researchers for very specific strands of RNA, and they can not target (or alter in anyway) your DNA. Antisense oligonucleotides only target RNA.
This video provides a good explanation of antisense oligonucleotides:
Have antisense oligonucleotides been tested in models of Parkinson’s?
Yes, they have.
There have been several interesting research reports published. Most recently, this one:
 Title: Amido-bridged nucleic acid (AmNA)-modified antisense oligonucleotides targeting α-synuclein as a novel therapy for Parkinson’s disease.
Title: Amido-bridged nucleic acid (AmNA)-modified antisense oligonucleotides targeting α-synuclein as a novel therapy for Parkinson’s disease.
Authors: Uehara T, Choong CJ, Nakamori M, Hayakawa H, Nishiyama K, Kasahara Y, Baba K, Nagata T, Yokota T, Tsuda H, Obika S, Mochizuki H.
Journal: Sci Rep. 2019 May 21;9(1):7567.
PMID: 31110191 (This report is OPEN ACCESS if you would like to read it)
In this study the researchers designed antisense oligonucleotides to reduce levels of Parkinson’s-associated alpha synuclein protein.
What is alpha synuclein?
Alpha synuclein sounds like a distant galaxy, but it is one of the most common proteins in our brains. It makes up about 1% of all the protein in a neuron. When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure.
When it is first produced, alpha synuclein will look something like this:
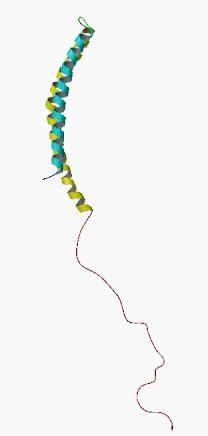
Alpha synuclein. Source: Wikipedia
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). It is believed that alpha synuclein has certain functions as a monomer, but may also have specific tasks as an oligomer.
In Parkinson’s, alpha synuclein will also misfold and aggregate together to form amyloid fibrils.

Microscopic images of monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein that aggregate together, and then go on to form what we call Lewy bodies.
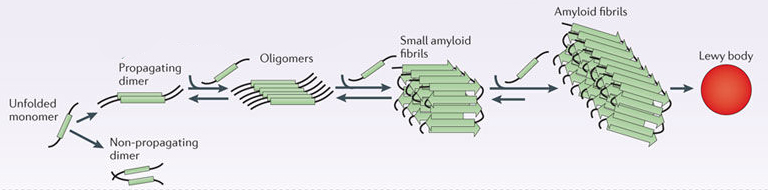 Parkinson’s associated alpha synuclein. Source: Nature
Parkinson’s associated alpha synuclein. Source: Nature
A Lewy body is referred to as a cellular inclusion, as they are almost always found inside the cell body. They are a characterisitic feature of the Parkinsonian brain.
 A photo of a Lewy body inside of a neuron. Source: Neuropathology-web
A photo of a Lewy body inside of a neuron. Source: Neuropathology-web
The researchers found that their alpha synuclein targeting antisense oligonucleotides efficiently reduced levels of both alpha synuclein RNA and protein – in human cells grown in culture and in the brains of mice.
Importantly, the treatment was able to significantly reduce levels of alpha synuclein and rescue the neurological defects observed in mice that have been genetically engineered to produce high levels of normal human alpha synuclein protein.
 Antisense oligonucleotide (ASO) treatment reduces alpha synuclein. Source: PMC
Antisense oligonucleotide (ASO) treatment reduces alpha synuclein. Source: PMC
And similar results has been independently produced by other research groups:
 Title: Selective α-Synuclein Knockdown in Monoamine Neurons by Intranasal Oligonucleotide Delivery: Potential Therapy for Parkinson’s Disease.
Title: Selective α-Synuclein Knockdown in Monoamine Neurons by Intranasal Oligonucleotide Delivery: Potential Therapy for Parkinson’s Disease.
Authors: Alarcón-Arís D, Recasens A, Galofré M, Carballo-Carbajal I, Zacchi N, Ruiz-Bronchal E, Pavia-Collado R, Chica R, Ferrés-Coy A, Santos M, Revilla R, Montefeltro A, Fariñas I, Artigas F, Vila M, Bortolozzi A.
Journal: Mol Ther. 2018 Feb 7;26(2):550-567.
PMID: 29273501 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers gave mice antisense oligonucleotides (via nasal administration) targeting alpha synuclein. This treatment reduced levels of alpha synuclein RNA and protein (by approximately 20-40%) in many areas of the brain that are affected by Parkinson’s (such as the substantia nigra where the dopamine neurons reside) without adversely affecting the mice.
The investigators now plan to test this nasally administered antisense oligonucleotide treatment in an alpha synuclein-based animal model of Parkinson’s.
Another research group, however, have already reported that antisense oligonucleotide-based reductions of alpha synuclein can help in a different genetic model of Parkinson’s:
 Title: G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons.
Title: G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons.
Authors: Volpicelli-Daley LA, Abdelmotilib H, Liu Z, Stoyka L, Daher JP, Milnerwood AJ, Unni VK, Hirst WD, Yue Z, Zhao HT, Fraser K, Kennedy RE, West AB.
Journal: J Neurosci. 2016 Jul 13;36(28):7415-27.
PMID: 27413152 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers used antisense oligonucleotides targeting Parkinson’s associated alpha synucleina in a LRRK2-based genetic model of Parkinson’s.
What is LRRK2?
Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’) – also known as ‘Dardarin‘ (from the Basque word “dardara” which means “trembling”) – is an enzyme that has many functions within a cell – from supporting efforts to move things around inside the cell to helping to keep the power on (involved with mitochondrial function).

The many jobs of LRRK2. Source: Researchgate
The LRRK2 gene is made up of many different regions. Each of those regions is involved with the different functions of the eventual protein. As you can see in the image below, the regions of the LRRK2 gene have a variety of different functions:
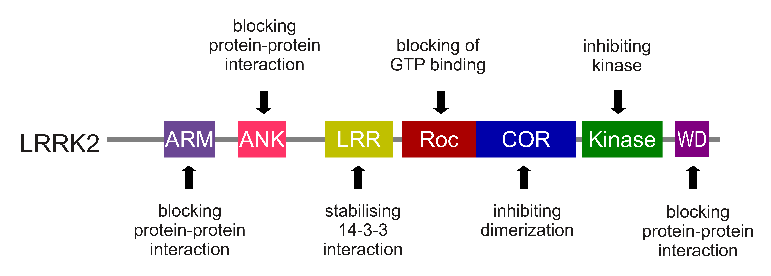
The regions and associated functions of the LRRK2 gene. Source: Intechopen
Genetic errors or variations within the LRRK2 gene are recognised as being some of the most common with regards to increasing ones risk of developing Parkinson’s (LRRK2 variants are present in approximately 1-2% of all cases of Parkinson’s).

The structure of Lrrk2 and where various mutations lie. Source: Intech
As the image above suggests, mutations in the PARK8 gene are also associated with Crohn’s disease (Click here and here for more on this) – though that mutation is in a different location to those associated with Parkinson’s. And one particularly common Parkinson’s-associated LRRK2 mutation – called G2019S – is also associated with increased risk of certain types of cancer, especially for hormone-related cancer and breast cancer in women – Click here to read more about this. If you have a G2019S mutation, no reason to panic – but it is good to be aware of this association and have regular check ups.
The G2019S mutation (the name designates its location on the gene) is the most common LRRK2 mutation. In some populations of people it can be found in 40% of people with Parkinson’s (Click here to read more about this). But what is interesting about this mutation is that it gives rise to a LRRK2 enzyme that is hyperactive.
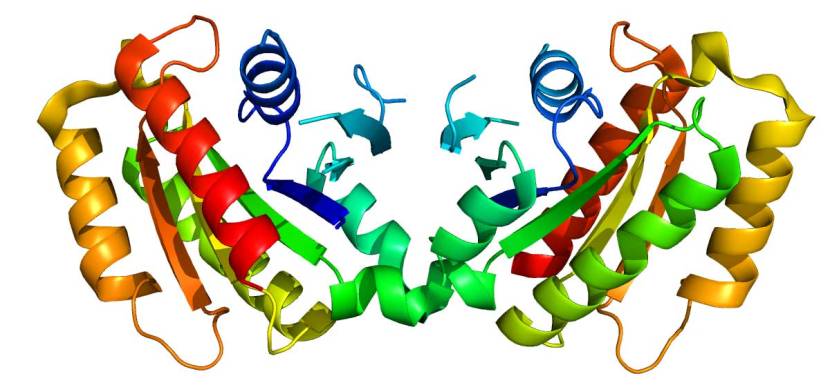
The structure of LRRK2 protein. Source: Wikipedia
As a protein, LRRK2 interacts with many different types of other proteins, and you can imagine that in a finely balanced environment like the cells that a mutant ‘hyperactive’ form of LRRK2 is going to cause problems. The consequences of this constantly active form of LRRK2 protein is believed to be influential in the cell death in LRRK2-associated Parkinson’s.
And this is where antisense oligonucleotides are being tested.
The researchers reported that high levels of G2019S-LRRK2 protein (in both cultured neurons and dopamine neurons in rats) increases the aggregation of alpha synuclein. Curiously, they found that high levels of normal LRRK2 (not hyperactive G2019S-LRRK2) reduced the abundance of alpha synuclein.
Reducing alpha synuclein levels with antisense oligonucleotides significantly reduced protein aggregation in cells with high levels of G2019S-LRRK2. These results suggest not only reducing alpha synuclein with antisense oligonucleotides is possible, but also that LRRK2 influences the aggregation of this protein.
And the researchers have followed up this work by exploring the use of antisense oligonucleotides targeting LRRK2:
 Title: LRRK2 Antisense Oligonucleotides Ameliorate α-Synuclein Inclusion Formation in a Parkinson’s Disease Mouse Model.
Title: LRRK2 Antisense Oligonucleotides Ameliorate α-Synuclein Inclusion Formation in a Parkinson’s Disease Mouse Model.
Authors: Zhao HT, John N, Delic V, Ikeda-Lee K, Kim A, Weihofen A, Swayze EE, Kordasiewicz HB, West AB, Volpicelli-Daley LA.
Journal: Mol Ther Nucleic Acids. 2017 Sep 15;8:508-519.
PMID: 28918051 (This report is OPEN ACCESS if you would like to read it)
In this study, the investigators were seeking to test whether antisense oligonucleotides targeting LRRK2 could reduce LRRK2 protein levels and the aggregation of alpha synuclein in mice – a “two birds, one stone” sort of thing. Mice were injected with alpha synuclein fibrils – the toxic form of the aggregating protein – to encourage further alpha synuclein aggregation in the brain. They were then injected with LRRK2 antisense oligonucleotides.
The animals treated with the LRRK2 antisense oligonucleotides exhibited less neurodegeneration than mice treated with a control oligonucleotide. These findings suggest that a reduction of endogenous levels of normal LRRK2 also reduces the formation of alpha synuclein aggregates.
Most importantly, this study points toward the use of LRRK2 antisense oligonucleotides as a potential therapeutic strategy for Parkinson’s. And by targeting this therapy to the brain, it could reduce possible
adverse side effects in peripheral tissues which have been reported using LRRK2 inhibitor drugs.
Is any of this preclinical research being targetted towards clinical testing in Parkinson’s?
So this is where the tale gets really interesting.
Recently a clinical trial was registed on the ClinicalTrials.gov website – Click here to read more about the details of this study.
One of the companies involved in this trial has been involved with some of the Parkinson’s research discussed above. That company is called Ionis Pharmaceuticals.
It was previously known as Isis Pharmaceuticals, but it changed this name in December 2015 when the label ISIS took on negative connotations.
The second company in this new clinical trial is the pharmaceutical company Biogen.
A rather potent combination.
And what is the new clinical trial all about?
The two companies registered a Phase I clinical trial this week to evaluate BIIB094.
What is BIIB094?
Exactly what BIIB094 is is currently not clear.
There is no reference to it on either company’s website. But drug analyst website suggests it is an antisense oligonucleotide approach. Also, given the companies involved and the fact that the treatment will be “administered via intrathecal injection to participants with Parkinson’s” and given that participants will be “LRRK2” and “Non-LRRK2”, I am making the (fairly safe) assumption that BIIB094 is an antisense oligonucleotide targetting LRRK2.
The trial is a Phase I clinical trial to evaluate the safety and tolerability of single and multiple doses of BIIB094
The secondary objective of the clinical trial will be evaluate the pharmacokinetic profile of BIIB094.
What does that mean? Pharmacokinetic profile?
Early clinical trials focus on safety and pharmacokinetics/pharmacodynamics. Pharmacodynamics is the effect that drugs have on the body – what happens to the body when a treatment is administered. Pharmacokinetics, on the other hand, is the study of what happens to the treatment when it is inside the body. Pharmacokinetics explores the way in which therapies move through the body, taking into account absorption, distribution, metabolism and excretion.
This new trial will involve 62 participants (all diagnosed with Parkinson’s in the last 7 years), evaluating single and multiple doses of BIIB094. There are few other details of the study at present, and while it has not yet started, it is expected to be completed in early 2022.
Is this the only biotech developing antisense oligonucleotides for Parkinson’s?
Another company is also exploring antisense oligonucleotide for Parkinson’s.
That company is called nLife Therapeutics.
 The company was involved in the nasal administration antisense oligonucleotide research discussed above and it has a treatment called NLF-PD-1233, which has recieved funding support from the Michael J Fox Foundation (Click here to read more about this).
The company was involved in the nasal administration antisense oligonucleotide research discussed above and it has a treatment called NLF-PD-1233, which has recieved funding support from the Michael J Fox Foundation (Click here to read more about this).
Are there any other clinical trials for neurodegenerative conditions testing antisense oligonucleotide?
You may recall last year (2018), that there was a lot of excitement in the Huntington’s community about the results of a clinical trial testing a new therapy.
What is Huntington’s?
Huntington’s disease is a neurodegenerative condition which results in individuals losing all inhibition of movement, giving rise to appearance of chorea – jerky, random, and uncontrollable movements (similar to dykinesias).
Huntington’s disease is a genetic condition, caused by an increase in a region of DNA inside the Huntingtin (Htt) gene.
This regions is made up of CAG repeats. In normal healthy humans, we usually have up to 30 repeats of CAG. If you have more than 40 CAG repeat, you are definitely going to develop Huntington’s disease. It is a simple diagnostic test.

Expansion of CAGs in the Huntingtin gene. Source: NIST
The expansion of the CAGs in the Huntingtin gene results in a mutant form of the Huntingtin protein. This mutant Huntingtin protein clusters and forms aggregates, and is believed to be associated with the cell death and clinical features observed in people suffering from Huntinton’s disease.
Last year, the results of a clinical trial were announced.
You may recall this as it caused a lot of media attention, with headlines like “Has a groundbreaking drug trial found a cure for Huntington’s?“. The trial was evaluating IONIS-HTTRx (also called RG6042) as a potential treatment for Huntington’s.
IONIS-HTTRx is an antisense oligonucleotide.
It is designed to stick to a abnormal Huntingtin RNA, and this action reduces the amount of mutant Huntingtin protein that is produced by each cell.
The treatment was originally developed by Ionis Pharmaceuticals, who conducted a Phase I/IIa clinical trial of IONIS-HTTRx in 2017 (Click here to read more about this). That trial involved 46 people with Huntington’s, who were recruited across nine centers in the UK, Germany, and Canada. The participants in the study were randomly assigned to receive either IONIS-HTTRx (in increasing doses) or a placebo treatment.
Recently, the results of that trial have been published:
 Title: Targeting Huntingtin Expression in Patients with Huntington’s Disease.
Title: Targeting Huntingtin Expression in Patients with Huntington’s Disease.
Authors: Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, Wild EJ, Saft C, Barker RA, Blair NF, Craufurd D, Priller J, Rickards H, Rosser A, Kordasiewicz HB, Czech C, Swayze EE, Norris DA, Baumann T, Gerlach I, Schobel SA, Paz E, Smith AV, Bennett CF, Lane RM.
Journal: N Engl J Med. 2019 May 6. [Epub ahead of print]
PMID: 31059641
The results of this study show that the treatment was safe and well tolerated and that a dose-dependent reductions in concentrations of mutant huntingtin protein were observed. Whether this reduction actually slows or changes the course of the condition is yet to be determined in a longer and larger study.
In order to do this, Ionis formed a partnership with the pharmaceutical company Roche in 2013, and then in late 2017 the company licensed IONIS-HTTRx to Roche. This means that Roche is solely responsible for the further development and commercialisation of the therapy.
A Phase III clinical trial is currently recruiting 660 people with Huntington’s (Click here to read more about this). The study will be evaluating the efficacy, safety, and effects of IONIS-HTTRx using various biomarkers (compared to placebo). Participants are being divided into three groups. One group will receive IONIS-HTTRx every four weeks, one group will receive IONIS-HTTRx every eight weeks (receiving placebo at alternate weeks), and one group will receive placebo only every four weeks. The study is recruiting patients at 26 locations across the U.S. and Canada, and will involve 2 years of treatment.
A lot of hopes are riding on that trial.
Another neurodegenerative condition that is being targetted with antisense oligonucleotides is familial amyotrophic lateral sclerosis (ALS or motor neuron disease).
What is familial amyotrophic lateral sclerosis?
Amyotrophic lateral sclerosis (or ALS), also known as Lou Gehrig’s disease and motor neuron disease, is a neurodegenerative condition in which the neurons that control voluntary muscle movement die. The condition affects 2 people in every 100,000 each year, and those individuals have an average survival time of two to four years.

ALS in a nutshell. Source: Walkforals
About 90 to 95 percent of ALS cases are sporadic, but a small number are called ‘Familial ALS’ as there is a genetic variant associated with their occurence. Genetic mutations in any of the C9orf72, SOD1, TARDBP, and FUS genes can increase an individuals risk of developing familial ALS (Click here to read more about this).
Biogen and Ionis Pharmaceuticals – the two biotech firms behind the LRRK2-Parkinson’s clinical trial discussed above – are currently testing Tofersen (also known as BIIB067 and IONIS-SOD1Rx), which is an antisense oligonucleotide approach targeting SOD1 RNA. The results should be reported next year (Click here to read more about this trial).
And thus, there is a quite a bit of clinical research going on for antisense oligonucleotides in neurodegenerative conditions.
So if antisense oligonucleotides work in Huntington’s and ALS, they might work for Parkinson’s?
Well, firstly it is NOT clear that “antisense oligonucleotides work in Huntington’s and ALS”.
At present, all we know – based on Phase I results in Huntington’s – is that these treatments appear to be safe and well tolerated. There is yet to be any indication that these treatments are disease modifying.
As to whether antisense oligonucleotides could work in Parkinson’s, this too is unclear. Huntington’s is a genetic disorder so antisense oligonucleotides may be a good treatment approach. Both ALS and Parkinson’s have less of a genetic component, so the rationale for antisense oligonucleotides is less clear.
Still it sounds very encouraging. What are we missing?
It is encouraging, but it is important to understand that DNA-based therapies faced significant challenges.
For example, when antisense oligonucleotides are injected into the body, they must avoid being broken down before getting into a cell and reaching their actual target. We produce high levels of enzymes called nucleases, whose sole function is to digest nucleic acids like antisense oligonucleotides. Thus, to be effective, antisense oligonucleotides need to be engineered (chemically modified to resist digestion).
Also, to be effective, antisense oligonucleotides must bind to their target RNA both tightly and with great specificity. Because they are highly charged molecules, antisense oligonucleotides can also bind to RNAs that they are not supposed to. This can affect the levels of other proteins, which could give rise to off-target effects and toxicity issues.
While the results of some of the new antisense oligonucleotide therapies are very exciting, caution should still be a priority.
Indeed. So, summing up time?
Not just yet.
There is another aspect of this tale that needs to be considered:
Nusinersen – which we discussed at the top of this post – is one of the most expensive treatments in the world. According to Wikipedia, it costs US$750,000 in the first year and US$375,000 annually after that. After long and protracted negotiations, only recently the NHS in the UK has begun to supply Nusinersen (Source).
And more recently, the Pharmaceutical company Novartis has been given regulatory approval for a one-time antisense oligonucleotide-based treatment called Zolgensma. But this treatment comes with a price tag of US$2.125 million. The hope is that costs will come down over time, but such trends do make one weary about getting too excited about new developments antisense oligonucleotide therapies.
Especially for treatment targets like LRRK2 where potentially cheaper drug alternatives are also being developed (Click here to read a previous SoPD post about this).
So what does it all mean?
In 1978, two scientists demonstrated that a single-stranded antisense oligonucleotide could inhibit viral replication in a laboratory experiment. This finding generated a lot of excitement at the time, and what followed was the usual trial and error process of taking a new technology to the clinic. Now after decades of trying, it appears that antisense oligonucleotides are finally starting to show their potential (and for the record, this statement has erroneously been made before).
 Source: Youtube
Source: Youtube
Given the recent success of novel antisense oligonucleotide therapies in other medical condition, it is only natural that this technology would be applied to Parkinson’s, and the initial preclinical results look encouraging. It is also rather exciting to see a new clinical trial for antisense oligonucleotides in Parkinson’s being registered in the last week. But as with all developments, expectations must be managed and we have to wait and see what will become of this new study.
Progress and activity is good, but we should have no expectations as to outcomes.
That said, fingers crossed!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, some of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies mentioned have requested that this material be produced, nor has the author had any contact with the companies. This post has been produced for educational purposes only.
The banner for today’s post was sourced from rdmag



11 thoughts on “Making sense of antisense”