|
Alpha synuclein is widely viewed as one of the bad guys in Parkinson’s. The clustering and aggregation of this protein is one of the main pathological hallmarks of the condition. Researchers led by scientists at the Scripps Research institute in Florida have developed a new drug-like compound that selectively prevents the production of alpha synuclein protein. They have called their new compound “synucleozid“. In today’s post, we will look at what alpha synuclein is, what synucleozid does, and how this approach could potentially help in treating Parkinson’s.
|
In a recent SoPD post, we discussed research focused on levodopa-induced dyskinesias which was led by scientists from the Scripps Research Institute (Click here to read that post). The Scripps is the largest private, not-for-profit medical research facility in the United States and among the largest in the world.
It is headquartered in La Jolla, California, but interesting fact: It has a sister facility in Jupiter, Florida.
 Scripps Research in Jupiter. Source: Weitz
Scripps Research in Jupiter. Source: Weitz
Officially opened on February 26th 2009, the establishment of the Scripps Florida campus was made possible by a one-time US$310 million federal economic development fund. The institute now survives on research grants, gifts, and contracts. In the future, some of the funding may also come from royalties generated by intelluctual property based on medical discoveries made at the facilities.
And some of those medical discoveries may involve novel ways to treat Parkinson’s.
Really? Such as?
Recently a research team led by Professor Matthew Disney published a report that takes an interesting approach towards trying to tackle Parkinson’s.
Professor Matthew Disney. Source: Scripps
What does it involve?
It involves reducing levels of the Parkinson’s associated protein alpha synuclein.
What is alpha synuclein?
We discuss alpha synuclein a lot on this website – if you don’t want to read another description of this protein (I won’t be offended), just skip down to RECAP #1.
Alpha synuclein is one of the most common proteins in the brain (making up about 1% of the protein in neurons). The exact function of alpha synuclein is not well understood, but research suggests that it plays a role in multiple cellular functions – including being involved in neurotransmitter release.
But in Parkinson’s, something changes.
For some reason, in many cases of Parkinson’s alpha synuclein protein starts to cluster and clump together. And this “aggregated” form of alpha synuclein appears to become toxic.
The aggregation of alpha synuclein is believed to lead to the appearance of Lewy bodies.
What are Lewy bodies?
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
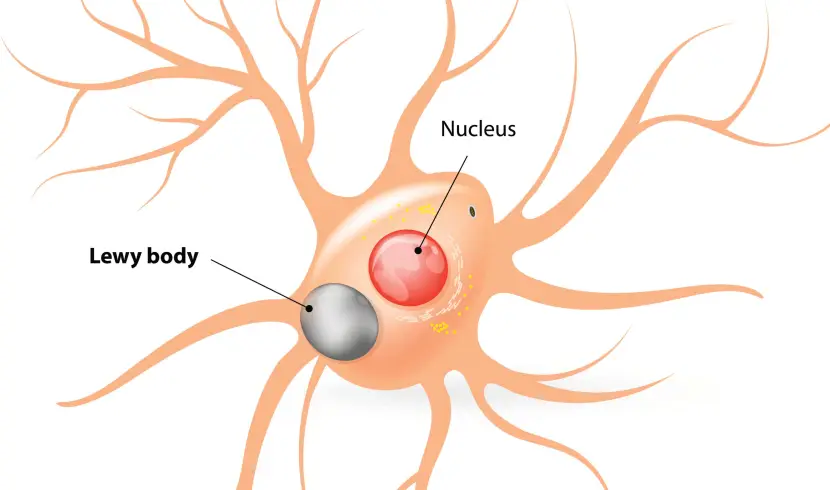 A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells – see the image below where alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (stained in brown; indicated by arrows). Source: Wikimedia
How does alpha synuclein protein become toxic?
When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure. Alone, it will look like this:
 Alpha synuclein. Source: Wikipedia
Alpha synuclein. Source: Wikipedia
By itself, alpha synuclein is considered a monomer, or a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of Monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein give rise to the aggregations of protein that we refer to as Lewy bodies:
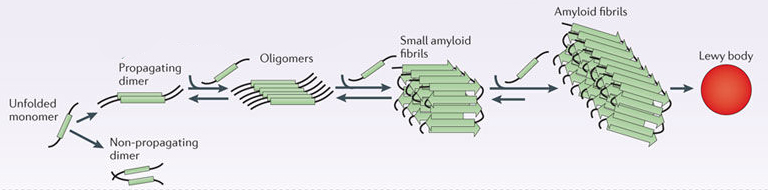 Parkinson’s associated alpha synuclein. Source: Nature
Parkinson’s associated alpha synuclein. Source: Nature
And it also believed that the oligomer and fibril forms of alpha synuclein protein may be being passed from cell to cell, and ‘seeding’ protein aggregation in new cells. And this is how the condition may be slowly progressing.
Is there any evidence of this transfer of the alpha synuclein protein?
So back in the 1990s, there were a series of clinical trials of cell transplantation conducted on people with Parkinson’s. The idea was to replace the cells that have been lost to the condition (Click here to read a previous post about cell transplantation). Many of the individuals who were transplanted in that study have now passed away (by natural causes) and their brains have been examined post-mortem.
One very interesting finding from the analysis of those brains is that some of the cells in the transplants have Lewy bodies in them (up to 10% of transplanted cells in one case – Click here to read the research report on that case).
 Above are photos of neurons from the post-mortem brains of people with Parkinson’s that received transplants. White arrows in the images above indicate lewy bodies inside transplanted cells. Source: The Lancet
Above are photos of neurons from the post-mortem brains of people with Parkinson’s that received transplants. White arrows in the images above indicate lewy bodies inside transplanted cells. Source: The Lancet
This finding suggested to researchers that somehow this neurodegenerative condition is being passed from the Parkinson’s affected brain to the healthy, young transplanted cells.
And researchers have proposed that the toxic form of the Parkinson’s-associated protein alpha synuclein may be the guilty party in this process (Click here to read more on this idea).
|
# RECAP #1: Alpha synuclein is a protein in the brain, that begins to cluster and aggregate in the Parkinsonian brain. The aggregated form of alpha synuclein is believed to be toxic and may be passed from cell to cell, providing an explanation of how the condition progresses. # |
So what did the Scripps researchers find?
They recently published this report describing their research:
 Title: Translation of the intrinsically disordered protein α-synuclein is inhibited by a small molecule targeting its structured mRNA.
Title: Translation of the intrinsically disordered protein α-synuclein is inhibited by a small molecule targeting its structured mRNA.
Authors: Zhang P, Park HJ, Zhang J, Junn E, Andrews RJ, Velagapudi SP, Abegg D, Vishnu K, Costales MG, Childs-Disney JL, Adibekian A, Moss WN, Mouradian MM, Disney MD.
Journal: Proc Natl Acad Sci U S A. 2020 Jan 3. [Epub ahead of print]
PMID: 31900363 (This report is OPEN ACCESS if you would like to read it)
In their study, the researchers were interested in levels of alpha synuclein.
Why are the levels of the protein so interesting?
Because some people carry multiple copies of the region of DNA that produce alpha synuclein, resulting in higher levels of the protein… and these individuals have a significantly increased risk of developing Parkinson’s.
So if you have more alpha synuclein you might develop Parkinson’s?
That’s what the evidence seems to suggest (Click here and here to read more about this).
Ok, so we should lower levels of alpha synuclein protein then?
And that is what Prof Disney and colleagues decided to try and do.
But rather than focusing on the protein, they targetted the translation of alpha synuclein.
Translation?!?
Translation is the process of creating proteins from an RNA template.
Que?
Ok, so you remember your high school science class when the adult at the front of the class was explaining biology 101? Like me, you may have slept through it, but hopefully you will remember a little something about the teachers saying that Deoxyribonucleic acid (or DNA) gives rise to Ribonucleic acid (or RNA), and RNA gives rise to protein. Yes?
This is the central dogma of biology. Do you remember it?

The basic of biology. Source: Youtube
DNA provides the instructions or the designs for making a protein, RNA is the template (or a photocopy of the designs) for making a protein, and protein is generally considered the functional end product in the process (but it is slightly more complicated than that).
The functional regions of DNA are referred to as genes. And it is each of these genes that help to keep you alive. The process of DNA providing the RNA template is called transcription, and this occurs inside the nucleus of a cell – where the DNA is safely stored. The process of RNA being used to produce protein is called translation, and this occurs outside of the nucleus.
For a good beginner introduction of the translation process, watch this video:
So the researchers wanted to target alpha synuclein RNA? Before the protein was actually produced?
Exactly.
But why? Why not just target the protein?
Because alpha synuclein is an intrinsically disordered protein – that is, a protein that lacks a fixed or ordered three-dimensional structure. Remember above, I wrote: When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure.
Being an intrinsically disordered, natively unfolded protein makes alpha synuclein difficult to target, due to a lack of clearly defined binding sites that are shared across all future versions of the protein.
And this lack of shared targets is why the researchers decided to target the alpha synuclein RNA.
|
# # RECAP #2: The production of protein from the RNA instructions is called translation. By inhibiting the translation of a particular protein, researchers can reduce the amount of that specific protein that a cell makes. Alpha synuclein is a intrinsically disordered protein, meaning that it is difficult to therapeutically target. This is why Prof Disney and colleagues chose to target alpha synuclein translation. # # |
I see. And how did they do this?
At the RNA level, alpha synuclein contains specific structures that can be targetted, such as an iron-responsive element.
You’re going to explain what this iron-responsive element thing is, right?
The iron responsive element (IRE in the image below) is a looped region of RNA that is present on many RNAs for proteins associated with iron related activities. The loop is bound by an iron regulatory protein (IRP) and this blocks translation of the RNA. Iron can bind to the iron regulatory protein, and this frees the RNA to undergo translation.
 Source: Researchgate
Source: Researchgate
Another reason why too much iron in Parkinson’s might be a bad thing, is that an excess of iron results in the IRP being bound by iron and alpha synuclein mRNA being translated – resulting in more alpha synuclein protein (Click here to read a previous post on iron).
So the researchers decided to target this iron responsive element of alpha synuclein?
Yes, they believed that small molecules that could target this tiny RNA structure may be of value as inhibitors of alpha synuclein translation.
They designed a collection of small molecules that bind to regions present in the alpha synuclein IRE, using a program that they developed called Inforna, which is described in this report:
 Title: Inforna 2.0: A Platform for the Sequence-Based Design of Small Molecules Targeting Structured RNAs.
Title: Inforna 2.0: A Platform for the Sequence-Based Design of Small Molecules Targeting Structured RNAs.
Authors: Disney MD, Winkelsas AM, Velagapudi SP, Southern M, Fallahi M, Childs-Disney JL.
Journal: ACS Chem Biol. 2016 Jun 17;11(6):1720-8.
PMID: 27097021 (This report is OPEN ACCESS if you would like to read it)
The researchers tested the identified alpha synuclein IRE-binding molecules and this effort led to the identification of the most effective compound, which they named Synucleozid.
 Synucleozid. Source: Medchemexpress
Synucleozid. Source: Medchemexpress
Further testing and validation studies demonstrated that synucleozid was physically binding to alpha-synuclein-encoding RNA at the IRE region, exhibiting high selectivity for the region, and also preventing translation.
One issue with targeting the IRE region, however, is that alpha synuclein is not the only RNA in the brain that contains an IRE region. So the researchers next tested how specifically synucleozid targets just the alpha synuclein IRE domain. They investigated three other RNAs that have IRE regions.
These included the RNA for Alzheimer’s associated amyloid precursor protein, prion disease-associated prion protein, and ferritin (which is a blood protein that is critical for iron homeostasis).
The researchers found that only ferritin exhibited a reduction in RNA levels when treated with synucleozid, but this was less than alpha synuclein levels.
Finally, the investigators wanted to know how synucleozid was blocking the translation of alpha synuclein RNA – by binding to the RNA, what cellular processes was it preventing? They found that synucleozid was stopping the ribosome from detecting the alpha synuclein RNA.
What are ribosomes?
Ribosomes are the molecular machines responsible for protein synthesis. They are minute particles consisting of two subunits (ingeniously called the ‘large subunit’ and the ‘small subunit’). The large subunit sits on top of the small subunit, and individual RNA templates are sandwiched between them.
 A ribosome. Source: KhanAcademy
A ribosome. Source: KhanAcademy
When a ribosome encounters an RNA, it binds to it and reads off the information it contains to build a string of amino acids that will eventually become a protein (or peptide).
This video explains the basics of ribosomes:
So synucleozid stops ribosomes from deteching the alpha synuclein RNA?
Yes, and by doing this, the synucleozid prevents the production (or translation) of the alpha-synuclein protein.
While their study thoroughly characterised the alpha synuclein-specific synucleozid, the researchers concluded that further research would be required before there could be any ‘translation’ of a different sort – the translation of preclinical research into human testing. But they were excited by the possibilities of this research for its wider applications. This technique could be used for developing molecules that can target RNA associated with other medical conditions, for example the neurodegenerative disease of Huntington’s.
|
# # # RECAP #3: The researchers designed a compound called synucleozid that targetted a particular region of alpha synuclein RNA and prevented it from being translated into protein. The investigators found that synucleozid was stopping the ribosome from detecting the alpha synuclein RNA. While development into clinical trials will require more research, the researchers are excited by the results, and are keen to apply this approach to other medical conditions involving protein accumulation. # # # |
So no sign of a synucleozid clinical trial any time soon?
No. While this research is interesting, this particular approach will require further development before exploring any potential medical utility.
But other RNA-based approaches are being explored for Parkinson’s.
For example, there are biotech companies already developing antisense oligonucleotide approaches for clinical testing in Parkinson’s.
What are antisense oligonucleotides?
Antisense oligonucleotides are small pieces of DNA or RNA that are designed to target and bind to very specific strands of RNA. By doing this, the antisense oligonucleotide blocks the ability of that piece of RNA to be used to make a protein. This reduces the amount of that protein in the cell and the inhibited piece of RNA is eventually disposed of.
 Source: Conversation
Source: Conversation
Each antisense oligonucleotide is designed by researchers for very specific strands of RNA, and they can not target (or alter in anyway) your DNA. Antisense oligonucleotides only target RNA.
We have previously discussed antisense oligonucleotides being developed for Parkinson’s (Click here to read that post), but the pharmaceutical company Biogen is very actively exploring this area for Parkinson’s.
 They have formed a major collaboration with a biotech company is called Ionis Pharmaceuticals.
They have formed a major collaboration with a biotech company is called Ionis Pharmaceuticals.
And together they are initiating a clinical trial to evaluate an antisense oligonucleotide treatment called BIIB094, which is designed to reduce levels of the Parkinson’s-associated protein LRRK2 ( Click here to read more about the details of this study).
But researchers from these two companies are also investigating antisense oligonucleotide treatment for alpha synuclein. They have recently made available a manuscript on the preprint database bioRxiv that presents some of the research they have conducted in this area:
 Title: Alpha-synuclein antisense oligonucleotides as a disease-modifying therapy for Parkinson’s disease
Title: Alpha-synuclein antisense oligonucleotides as a disease-modifying therapy for Parkinson’s disease
Authors: Cole TA, Zhao H, Collier TJ, Sandoval I, Sortwell CE, Steece-Collier K, Daley BF, Booms A, Lipton J, Welch M, Berman M, Jandreski L, Graham D, Weihofen A, Celano S, Schulz E, Cole-Strauss A, Luna E, Quach D, Mohan A, Bennett CF, Swayze EE, Kordasiewicz HB, Luk KC, Paumier KL
doi: https://doi.org/10.1101/830554 (This manuscript is OPEN ACCESS if you would like to read it)
In this study, the researchers found that in both rodent and primate models of Parkinson’s, alpha-synuclein RNA targetting antisense oligonucleotides were able to reduce the levels of alpha synuclein protein and improve outcomes.
In their concluding statement, the authors clearly state that antisense oligonucleotides designed against alpha synuclein “are progressing to the clinic”.
So we will keep an eye out for more news about this.
Another company exploring this approach is nLife Therapeutics in Spain.
 The company has a treatment called NLF-PD-1233, which has recieved funding support from the Michael J Fox Foundation (Click here to read more about this).
The company has a treatment called NLF-PD-1233, which has recieved funding support from the Michael J Fox Foundation (Click here to read more about this).
So what does it all mean?
RNA represents a critical step in the synthesis of proteins. By increasing the amount of a particular RNA floating around in the nucleus of a cell, one can increase the levels of the associated protein. Alternatively, by inhibiting a specific RNA, the levels of the associated protein can be reduced.
Therapies targetting specific disease-reated RNAs are now being developed for Parkinson’s. Recently researchers have developed a novel compound called synucleozid, which reduces the levels of alpha synuclein RNA/protein. While there is still a great deal of research to be done before this (or a synucleozid-like molecule) will be allowed to enter clinical trials, it is a remarkable achievement to have successfully demonstrated that such a molecule is possible.
With all kinds of alpha synuclein reducing approaches being developed for Parkinson’s (think immunotherapy, small molecules, gene therapy, antisense oligonucleotides, etc), what we require now is a clinical proof of principle demonstration that targetting this protein will have an impact on slowing the progression of Parkinson’s.
Hopefully we will learn more about this in the not so distant future.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, some of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies mentioned have requested that this material be produced, nor has the author had any contact with the companies. This post has been produced for educational purposes only.
The banner for today’s post was sourced from MickeyBlog



Simon wrote:
“… there is still a great deal of research to be done before this (or a synucleozid-like molecule) will be allowed to enter clinical trials …”
Things seem to be moving fast. Annovis Bio’s Phase 2a trial of Posiphen (ANVS401) is now recruiting PwPs (USA).
PNT article: First Patients Dosed in Clinical Trial Investigating ANVS401 as Treatment for Parkinson’s and Alzheimer’s
https://parkinsonsnewstoday.com/2020/09/03/first-patients-dosed-clinical-trial-anvs401-treatment-parkinsons-alzheimers/
LikeLike
Things continue to move fast. Annovis Bio’s Phase 3 trial of Buntanetap (Posiphen/ANVS401) is now recruiting PwPs (USA).
PNT article: First Patient Dosed in Phase 3 Trial of Buntanetap for Early Parkinson’s
https://parkinsonsnewstoday.com/news/first-early-stage-parkinsons-patient-dosed-phase-3-trial-buntanetap/
LikeLike
Annovis Bio’s Phase 3 trial of Buntanetap (Posiphen/ANVS401) has now completed recruitment (of 520 PwPs in USA and EU).
“The study is expected to conclude in November [2023] with top-line assessment data available by the end of the year.”
https://www.prnewswire.com/news-releases/annovis-bio-announces-completion-of-phase-iii-parkinsons-disease-treatment-enrollment-at-record-pace-301845622.html?tc=eml_cleartime
LikeLike
Attack of the Synucleozid-2.0!
MedicalXpress article: Targeting the mRNA of ‘undruggable’ proteins in the fight against Parkinson’s disease.
https://medicalxpress.com/news/2024-01-mrna-undruggable-proteins-parkinson-disease.html?utm_source=nwletter&utm_medium=email&utm_campaign=daily-nwletter
LikeLike