|
# # # # A Parkinson’s-focused biotech company called Enterin has had a very busy start to the new year, with publication of some interesting preclinical research and the announcement of Phase II clinical trial results. The clinical trial results met both the primary and secondary endpoints (the pre-determined measures of whether the treatment is effective) indicating a successful study, and the preclinical result provides new potential insights into the functions of the Parkinson’s-associated protein, alpha synuclein. In today’s post, we will discuss both the clinical trial results and the preclinical work, and consider what this means for our understanding of Parkinson’s. # # # # |
 Source: Discovery
Source: Discovery
In scientific nomenclature, they are referred to as Squalus acanthias.
Many people call them ‘Spurdogs’. Or ‘Mud sharks’. Or even ‘Piked dogfish’.
But they are more commonly known as spiny dogfish.
Source: X-ray Mag
Fun facts about spiny dogfish:
- They live in the shallow saltwater habitats of the North Pacific and the North Atlantic oceans
- The females are longer (49 inches or 124 cm) than the males (39 inches or 99 cm)
- They have two dorsal fins, both with venomous spines (hence the name)
- A pregnant females will have an average litter of 6 pups
- They have very long gestation periods – up to 24 months!
- The average lifespan ranges between 20 and 24 years
- Spiny dogfish are very fast swimmers – able to swim at about 6.2 feet/s (1.9 m/s)
- They have a special organ called the ‘Ampullae of Lorenzini‘ which they use to detect the electric field generated by their prey.
- They have a very keen sense of smell and two-thirds of their brain is involved in their sense of smell.
Oh, and they are extremely robust when it comes to infection.
Seriously, they never get sick, which is fascinating given that they have a relatively “primitive” immune system (Click here to read more on this).
Very interesting. But what does any of this have to do with Parkinson’s?
Well, it’s that strong anti-infection feature that made these animals particularly interesting to researchers, who then noticed an intriguing connection to Parkinson’s.
Back in the early 1990s, a team of researchers – led by Prof Michael Zasloff – were interested in determining what made spiny dogfish so resistant to infection, and they discovered that a major component of the strong immune response was attributable to a single protein.
They published their research in 1993: Title: Squalamine: an aminosterol antibiotic from the shark.
Title: Squalamine: an aminosterol antibiotic from the shark.
Authors: Moore KS, Wehrli S, Roder H, Rogers M, Forrest JN Jr, McCrimmon D, Zasloff M.
Journal: Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1354-8.
PMID: 8433993 (This report is OPEN ACCESS if you would like to read it)
When analysing the tissue of the spiny dogfish, the researchers discovered a water-soluble antibiotic that exhibited potent anti-bacterial activity against both Gram-negative and Gram-positive bacteria.
They call this protein “squalamine”.
What is Squalamine?
Squalamine is steroid with a wide range of antimicrobial activity. Steroids are used in medicine as treatments for certain inflammatory conditions.
 The structure of squalamine. Source: Wikipedia
The structure of squalamine. Source: Wikipedia
So how is squalamine related to Parkinson’s?
Well, in 2017, Prof Zasloff and a group of international collaborators but published some interesting research suggesting an additional property for squalamine.
This is the research article that was published:

Title: A natural product inhibits the initiation of α-synuclein aggregation and suppresses its toxicity
Authors: Perni M, Galvagnion C, Maltsev A, Meisl G, Müller MB, Challa PK, Kirkegaard JB, Flagmeier P, Cohen SI, Cascella R, Chen SW, Limboker R, Sormanni P, Heller GT, Aprile FA, Cremades N, Cecchi C, Chiti F, Nollen EA, Knowles TP, Vendruscolo M, Bax A, Zasloff M, Dobson CM.
Journal: PNAS 2017 Feb 7;114(6):E1009-E1017.
PMID: 28096355 (this article is OPEN ACCESS if you would like to read it)
In this study, the researchers discovered that squalamine is extremely efficient at blocking the aggregation of alpha synuclein.
Remind me again: What is alpha synuclein?
We talk about alpha synuclein a lot on this website. It is one of the most common proteins in the human brain, and many believe that it is centrally involved with the neurodegeneration associated with Parkinson’s.
The effects of aggregated Alpha Synuclein protein in a neuron. Source: R&D
Alpha synuclein has different functions in the brain – particularly in terms of communication between neurons. But in the Parkinsonian brain, for reasons that are still unknown to us alpha synuclein starts to clump (or ‘aggregate’) together. And this aggregation is believed to lead to the appearance of Lewy bodies.
What are Lewy bodies?
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies). They are a classical hallmark of the brain of someone affected by Parkinson’s.
A Lewy body within a neuron. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells. In the image below, alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (stained in brown; indicated by arrows). Source: Wikimedia
When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that it does not really have a defined structure. When it is first produced, alpha synuclein will look something like this:
Alpha synuclein. Source: Wikipedia
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of Monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein give rise to the aggregations of protein that go on to form the Lewy bodies we mentioned above:
Parkinson’s associated alpha synuclein. Source: Nature
Now, given this process, and its association with a neurodegenerative condition like Parkinson’s, a lot of effort is being put into identifying agents that can reduce this aggregation of alpha synuclein protein. It is hoped that by limiting this activity, we may be able to slow or stop completely the progression of the condition.
I see. So Prof Zasloff and colleagues discovered that squalamine can prevent alpha synuclein aggregation?
Yes. They treated human cells (that produce too much alpha synuclein, which ultimately kills them) in culture with squalamine and they observed an almost complete suppression of the toxic effect of aggregated alpha synuclein.
Next, the researchers looked at the effects of squalamine in a microscopic worm called Caenorhabditis elegans.

Caenorhabditis elegans – cute huh? Source: Nematode
These tiny creatures are widely used in biology because they can be easily genetically manipulated and their nervous system is very simple and well mapped out (they have just 302 neurons and 56 glial cells!). The particular strain of Caenorhabditis elegans used in their experiments produced enormous amounts of alpha synuclein, which if left untreated resulted in muscle paralysis in these animals.
By treating the worms with squalamine, the researchers observed a dramatic reduction of alpha synuclein protein aggregating and an almost complete elimination of the muscle paralysis. In addition, they noted a reduction in the cellular damage caused by the aggregation of alpha synuclein.
The researchers concluded their study by suggesting that “squalamine could be a means of therapeutic intervention in Parkinson’s”.
A recent follow-up study that was published last year replicated the initial Caenorhabditis elegans results and expanded on them – identifying that squalamine has a limited effect on alpha synuclein protein with a A30P mutation (Click here to read more about this).
Additional recent research has provided insights into how squalamine might be having its effect. Squalamine and related derivatives appear to displace alpha synuclein from the membrane of cells, limiting the potential for the initiating events of aggregation (Click here to read some of that research).
|
# RECAP #1: Squalamine is a protein with potent anti-bacterial properties, that was discovered in the tissue of spiny dogfish. Subsequent research has found that squalamine also exhibits strong inhibition against Parkinson’s-associated aggregation of alpha synuclein protein. # |
Has squalamine ever been tested in humans?
A Philadelphia-based biotech firm called Enterin Inc. has been developing a synthetic version of squalamine called ENT-01.
This company was set up by Prof Zasloff and colleagues to explore the potential of ENT-01 in Parkinson’s and related conditions.
In 2017, the company initiated a Phase I/2 clinical trial – called the RASMET study – to evaluate the safety and tolerability of ENT-01 as a treatment for constipation in Parkinson’s (Click here to read more about that study).
According to the company, neither squalamine or ENT-01 are readily absorbed into the bloodstream. But ENT-01 does act locally in the gut, interacting with the enteric nerve cells in the lining of the gut wall. The company hopes that this treatment will stimulate gut motility and alter neural signaling from the gut to the brain.
The results of the RASMET study have now been published:
 Title: Targeting neurons in the gastrointestinal tract to treat Parkinson’s disease.
Title: Targeting neurons in the gastrointestinal tract to treat Parkinson’s disease.
Authors: Hauser RA, Sutherland D, Madrid JA, Rol MA, Frucht S, Isaacson S, Pagan F, Maddux BN, Li G, Tse W, Walter BL, Kumar R, Kremens D, Lew MF, Ellenbogen A, Oguh O, Vasquez A, Kinney W, Lowery M, Resnick M, Huff N, Posner J, Ballman KV, Harvey BE, Camilleri M, Zasloff M, Barbut D.
Journal: Clin Park Relat Disord. 2019 Jul 2;1:2-7.
PMID: 34316590 (This report is OPEN ACCESS if you would like to read it)
The RASMET trial involved the recruitment of 44 people with Parkinson’s (who had suffered from constipation for over 6 months before starting the trial), and it was conducted in two stages: Stage 1 involved 10 participants and it sought to assess the safety, tolerability, and pharmacokinetics of single escalating doses of ENT-01 over a 30-60 day period. This part of the study involved a pre-treatment, 2-week run in period of assessment and it was followed by a 2-week wash-out period with further assessments.
Stage 2 of the study enrolled 34 people with Parkinson’s with constipation and they were administered ENT-01 daily (escalating every 3 days) to a dose that had a clear prokinetic effect (or else a maximum dose of 250 mg). This dose was then maintained for an additional 3–5 days, resulting in the participants being on a fixed dose for approximately 7 days. Following this treatment phase the participants were then followed up during a 2-week washout period.
What is a prokinetic effect?
A prokinetic effect is an enhancement of gastrointestinal motility – improving the frequency (or strength) of contractions in the small intestine, but without actually disrupting their rhythm.
In the RASMET study, pharmacodynamics (or the effect of the drug on the body) of ENT-01 were assessed along with safety and tolerability. Relative outcomes were compared within each patient and across groups. Frequency of bowel movements and other non-motor symptoms of Parkinson’s were also collected over the course of both parts of the study.
The primary outcome of the study (this is a predetermined measure of success) was the number of participants with treatment-related adverse events. This was used to determine the safety of the drug, and the results indicate that the drug was safe and well tolerated. The secondary outcome measure will be the frequency of bowel movements and this too indicated that the orally administered ENT-01 treatment improved bowel function in individuals with Parkinson’s.
The researchers also noted minimal absorption of the drug (as measured by blood assessment) suggesting that the observed improvements were the result of local stimulation on the nerves in the lining of the gut. And in their concluding comments, they mentioned that a “double-blind, placebo-controlled study is now ongoing“.
That “double-blind, placebo-controlled study” was called the ‘KARMET’ study. And the results of that study have very recently been shared in a press statement from the company.
What were the findings of the KARMET study? Were they positive?
KARMET was a Phase IIb randomized, placebo-controlled, double-blind study of ENT-01 involving 150 individuals with Parkinson’s. Following a 2-week baseline period, participants were stratified to high dose or low dose depending on baseline constipation severity & randomized to receive ENT-01 or placebo. They were treated and monitored for a 25-day period, then all placed on placebo for 2 weeks before going through a 4-week wash-out (Click here to read more about this study).
- A 2-week run-in period
- A 3-5 week escalating dose period to identify a prokinetic dose in the initial set of 10 patients
- A 1-week period of randomised dosing (placebo versus the previously identified pro-kinetic dose)
- A 2-week wash-out period.
The results of the KARMET study indicate that (once again) ENT-01 was found to be safe & well tolerated, with common adverse events being primarily gastrointestinal in nature.
The primary endpoint in the study – change in complete spontaneous bowel movement from baseline to the end of the 3-week treatment period – was met: The researchers observed that bowel movement was significantly better in the ENT-01 treatment group compared to placebo (p=0.0001). It is interesting to note that there was some maintenance of this effect in the washout phase of the study (the last 6 weeks of the graph below):
 Source: Enterin
Source: Enterin
In addition, all of the bowel-related secondary endpoints improved in the ENT-01 treatment group.
Interestingly, there was also a reduction in levels of psychosis (as measured by SAPS-PD) during 3 week study period, & effect persisted out to 6 weeks post termination of treatment (small numbers in this result, but trend is present)
 Source: Enterin
Source: Enterin
Motor scores (as determined by UPDRS III) were measured, but this was only done for safety reasons (the 3 week study was too short for any meaningful efficacy measures). The results indicated that there was no worsening of motor symptoms during the study for either treatment group.
Enterin is yet to announce what the next steps are in terms of clinical testing of ENT-01 for Parkinson’s, and they will probably now be seeking guidance from the US FDA regarding those next steps.
That said, Enterin has also been assessing ENT-01 in Parkinson’s Disease Dementia in a Phase I open label study (Source). This study was scheduled to complete in mid-2021, so we will hopefully also learn the results of this study at some point in 2022.
|
# # RECAP #2: A biotech company called Enterin Inc. has been developing a synthetic version of squalamine called ENT-01. They have been clinically testing this agent in people with Parkinson’s. The results of a recent Phase IIb clinical trial involving 150 individuals with Parkinson’s found that ENT-01 significantly improved features of constipation and also demonstrated interesting effects in terms of psychosis. # # |
So summing up time? What does it all mean?
No, we’re not finished yet.
You see, the researchers at Enterin (in collaboration with academic scientists) also recently published this report:
 Title: Alpha synuclein, the culprit in Parkinson disease, is required for normal immune function.
Title: Alpha synuclein, the culprit in Parkinson disease, is required for normal immune function.
Authors: Alam MM, Yang D, Li XQ, Liu J, Back TC, Trivett A, Karim B, Barbut D, Zasloff M, Oppenheim JJ.
Journal: Cell Rep. 2022 Jan 11;38(2):110090.
PMID: 35021075 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers were interested in how alpha synuclein could be playing a role in inflammation in the gut.
Alpha synuclein is involved with inflammation in the gut?
So back in 2017, Prof Zasloff and colleagues published this report:
 Title: A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity.
Title: A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity.
Authors: Stolzenberg E, Berry D, Yang D, Lee EY, Kroemer A, Kaufman S, Wong GCL, Oppenheim JJ, Sen S, Fishbein T, Bax A, Harris B, Barbut D, Zasloff MA.
Journal: J Innate Immun. 2017;9(5):456-463.
PMID: 28651250 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers analysed gut biopsies from children who had been diagnosed with gastric inflammation and also from intestinal allograft recipients who contracted norovirus.
The investigators found that the levels of alpha synuclein in the enteric nervous system of the upper gastrointestinal tract of pediatric patients positively associated with the degree of inflammation in the intestinal wall. The more alpha synuclein, the higher the levels of inflammation.
Interesting. But what is the “enteric nervous system”?
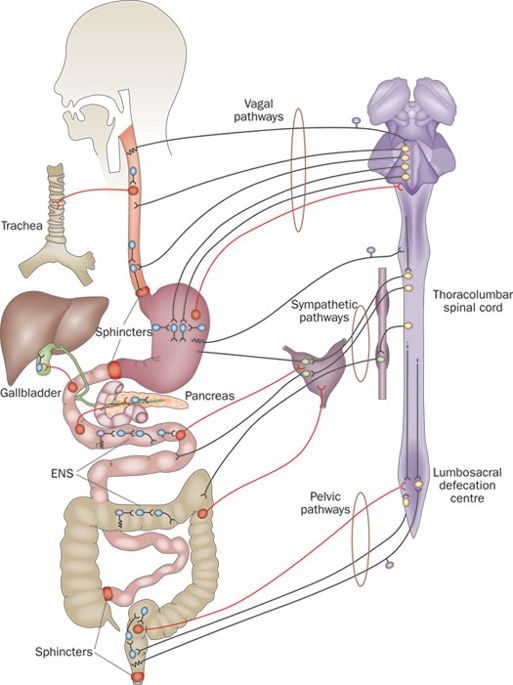
The nerves surrounding the gastrointestinal system are collectively known as the enteric nervous system:
Enteric nervous system. Source: Nature
The enteric nervous system consists of a mesh-like network of neurons that governs many aspects of function in the gastrointestinal tract, including the secretion of gastrointestinal enzymes.
So the researchers found that levels of alpha synuclein in this network of neurons around the gut correlated with the level of inflammation?
Exactly, and they also found that alpha synuclein has chemoattractant activity.
What is chemoattractant activity?
Chemoattractants are small messenger proteins that bind to receptors on the surface of leukocytes (types of immune cells that help the body fight infection), causing their activation and locomotion. Chemoattractants are a means of ‘attracting’ immune cells to the site of a problem.
Leukocyte movement towards higher concentrations of chemoattractants is referred to as chemotaxis.
So alpha synuclein can stimulate cells in the immune system?
Yep.
They found that both monomeric and oligomeric forms of the alpha synuclein have strong chemoattractant activity.
The investigators concluded that first study by proposing that alpha synuclein is produced within the enteric nervous system and it can influence intestinal inflammation. They also proposed that this could have implications for conditions associated with alpha synuclein, like Parkinson’s.
Ok, so that was the result of the previous study. What did the researchers find in their new study?
Well, to explore these findings further, the researchers used two groups of mice:
- normal mice, and
- genetically engineered mice that produce no alpha synuclein
 Source: Pinterest
Source: Pinterest
They next caused a bacterial infection in the guts of these two types of mice and looked at the response in terms of gastrointestinal inflammation.
In the normal mice, this infection caused increased levels of alpha synuclein, an infiltration of immune cells, and the production of inflammatory cytokines (these are another type of messenger protein that can help to stimulate an immune response).
Interesting. What happened in the mice that produce no alpha synuclein?
Well in contrast to the normal mice, when the investigators induced a bacterial infection in the gut of the genetically engineered mice that produce no alpha synuclein, it resulted in fewer immune cells invading the gut and lower levels (less than half) of proinflammatory cytokines being produced. And this effect appears to be related to activities during the early stages of the infection as the decreased levels were more evident in the first few hours of the experiment than at 24 hour after injection.
Next the researchers wanted to know how a protein like alpha synuclein (which functions inside of neurons) could be having this effect. When they collected tissue from the mice, the scientists found a marked increase in the concentration of alpha synuclein protein in the fluid of the collected tissue, indicating that the protein was being released by the cells that were producing it.
Released?
Yes. Secreted out into the extra-cellular world. And through a series of experiments, the researchers determined that the source of the released alpha synuclein was most likely the neurons of the enteric nervous system.
Next the researchers shifted their attention to the response of immune cells to this secreted alpha synuclein. By exploring different cell types (such as macrophages and dendritic cells), the investigators found that the released alpha synuclein had the potential to activate many of these types of immune cells.
And this finding indicated that alpha synuclein may function as an alarmin.
What is an alarmin?
Alarmins are proteins (or peptides) that are released by cells when stimulated by danger signals, and they help to galvanize immune cells – effectively engaging the adaptive immune system to establish an adequate defense.
To test the idea that alpha synuclein could be acting as an alarmin, the researchers abdominally injected alpha synuclein protein into mice and 4 hours later they analysed the immune cells in the area of the injection. They found a marked increase in these cells and their activation. So even in the absence of a bacterial infection, alpha synuclein was able to stimulate the immune system.
In their conclusions, the investigators stated that the study not only found that alpha synuclein production and secretion from gut neuronal cells was augmented during the bacterial infection, but also that alpha synuclein was critical to the immune/inflammatory response to the infection.
Quite a busy start to the year for the team at Enterin. Here at SoPD HQ, we look forward to learning what they next have to share.
So what does it all mean?
We have covered a wide set of topics in the post today: First, some encouraging clinical results, and then preclinical data that could provide new insights into a potential function of alpha synuclein. The implications for both are wide ranging.
It is interesting that we started this post with the discovery of a steroid-like molecule that had anti-inflammatory and anti-bacterial properties, and then discussed encouraging clinical trial results involving this molecule in people with Parkinson’s, before exploring preclinical data indicating a role for alpha synuclein in the inflammatory signaling of the immune system. Almost feels like a full circle.
One aspect that I like about the clinical part of the post today is that the biotech company Enterin is focused on a very specific aspect of Parkinson’s (that being gut function and constipation) which has relatively objective measures, rather than the wholistic idea of overall “disease modification” with broader clinical rating scales (such as UPDRS) as their endpoints. By narrowing in on something like constipation, the company will be able to test their drug relatively quickly and get it into the clinic more rapidly (if it is found to be safe and efficacious at Phase III). Once it is approved, of course, the company will be better placed to conduct further clinical evaluations to assess any additional potential benefits.
Meanwhile, the preclinical data – indicating that neurons may be secreting alpha synuclein in response to an infection or stressor, and this action is playing a role in the inflammatory activities of the immune system – could have important implications for ongoing clinical trials targeting both alpha synuclein and inflammation. Immunotherapy studies (which involve antibodies that target alpha synuclein protein) have been targeting the transfer of alpha synuclein from one cell to another in the brain, but based on these new results, this therapeutic approach could potentially be useful at dampening down inflammation, particularly in areas like the gut.
The start of the year is always a busy time for biotech companies as they share new data and goals/milestones for the next 12 months. Enterin has raised the bar in terms of sharing new data, and we now eagerly await their plans for taking ENT-01 forward for further late-stage clinical testing.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: Enterin is a privately owner company. The company has not requested that this material be produced, nor has the author had any recent contact with the company or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Enterin







Thank you so very much for your intelligent and informative posts and efforts to inform those of us impacted by Parkinson’s- directly or indirectly. I am so grateful.
>
LikeLike
Hi vjf47748,
Thanks for your comment and kind words. You are very welcome – glad you like the website.
Kind regards,
Simon
LikeLike
Does “gut function and constipation” include or effect gastroparesis – slow stomach emptying? 50to 70 to 100% (depending on the study one reads) of us need to know, if possible. Thanks
LikeLike
Alpha synuclein, the culprit in Parkinson disease, is required for normal immune function (Michael Zasloff et al.):
The opening sentence of the research paper states the following: “Alpha synuclein (aS) plays a key role in the pathogenesis of Parkinson disease (PD). The best evidence for this is that expression of multiple genes for aS results in severe inherited familial PD (Chartier-Harlin et al., 2004; Polymeropoulos et al., 1997; Singleton et al., 2003).”
I understand this to mean that too much alpha-syn (e.g. by duplication/triplication of the SNCA gene), can, in itself, cause PD.
In the Discussion section of the research paper they state the following: “… if these stimuli persist chronically, either due to repeated infection or a failure to effectively contain commensal bacteria, the stimulation of aS would be unremitting.”
I understand this to mean that chronically-persistent stimuli could result in too much alpha-syn.
I know this is very simplistic, but it seems to me that we might not need to discover “what happens mechanistically to the alpha synuclein that changes it from a protein that is just serving its normal function to a pathogenic alpha synuclein that is associated with disease” [1]. All that might be required is a way to prevent too much alpha-syn being produced (e.g. Annovis Bio’s approach, using Buntanetap/Posiphen).
[1] Puzzling Parkinson’s protein plays essential role in immunity, Meredith Wadman, Science Magazine, 12 Jan 2022.
LikeLike
Hi Simon, my dad was diagnosed with parkinsons. Your blog has been really helpful thank you. Is there any way to contact you via email for some advice? Just asking out of desperation.
LikeLike
Hi Sonya,
Thanks for your message. Sorry to hear about your Dad’s diagnosis. You can contact me at: scienceofparkinsons@gmail.com
Kind regards,
Simon
LikeLike
Thank you for your cler and enlightening post. I thinks phase 2b has been completed. How long do we need to see the product on the market?
LikeLike
I reckon it will be at least another 2 years, as they have yet to do a Phase 3 trial.
Also, they have recently announced a collaboration with Parkinson’s UK to conduct a new Phase 2 trial for PD-related dementia. This might stretch their resources and result in a delay for the PD-related constipation Phase 3 trial.
https://www.parkinsonsvirtualbiotech.co.uk/single-post/enterin-and-parkinson-s-uk-announce-research-collaboration-in-parkinson-s-dementia
LikeLike