|
Recently new research has been published that raises the question (again) as to whether there is something wrong with the immune system in Parkinson’s Researchers from Germany and San Diego (USA) have published data suggesting that a particular type of blood cell may be acting up in Parkinson’s, getting involved with the neurodegenerative process that characterises the condition. In their report they also found a clinically available treatment – called Secukinumab – that could reduce the effect. In today’s post, we will look at what lymphocytes are, how they may be playing a role in Parkinson’s, and explain how secukinumab could potentially aid us in the treatment of PD. |
Ouch! Source: CT
My 5 year old recently cut her leg, and there was a bit of blood. We patched her up with a plaster, but also took advantage of the moment to learn a little something about how the body works.
Me: Do you know what that red stuff is?
Little monster: It is blood?
Me: That’s right.
Little monster: Papa, where does blood come from?
That was when I got all excited, and pulled out my black board.
This was the answer I gave her:
Source: Wikipedia
The field of hematopoiesis (or blood formation) is absolutely fascinating.
You start off with a couple of small multi-potential hematopoietic stem cells (or a “Hemocytoblasts”) and – it given enough time – they can give rise to an entire blood system (this has actually been demonstrated in heavily irradiated mice – Click here to read more about this).
And as the image above suggests, there is quite a lineage of potential cells that these blood stem cell can generate.
What is blood?
Blood is a body fluid that animals use to deliver nutrients and oxygen to all of the various organs/tissues of the body and transport cellular waste. Blood accounts for approximately 7% of your body weight, and the average adult has a blood volume of roughly 5 litres (11 US pints).
What is blood made of?
There are three chief components of blood:
- Red blood cells
- White blood cells
- Plasma (92% water and 8% other stuff – think blood clotting proteins, waste, nutrients, etc)
By volume, the red blood cells constitute about 45% of whole blood, the plasma about 54.3%, and white cells about 0.7%.
Source: KhanAcademy
When it comes to the cellular parts of blood, one microliter of blood contains:
- Approximately 5 million erythrocytes, which are red blood cells which distribute oxygen around the body. These are small, bi-concave shaped cells that lack any nucleus, but this extra space allows for more hemoglobin – the critical protein involved in transportation of oxygen.
Source: KhanAcademy
- 200,000–500,000 thrombocytes, (also known as platelets) which take part in blood clotting. Thrombocytes are are not actually cells, but rather cell fragments. They are produced when large cells called megakaryocytes which each release 2-3000 platelets in their life time. Platelets are small (2-4 micrometers), lens-shaped structures that float around in the blood system waiting to help plug a rupture.
Source: KhanAcademy
- 5,000–10,000 leukocytes, which are the white blood cells that form the body’s immune system – chewing up and removing old or rogue cells or waste, as well as attacking any foreign or infectious agents.
For the rest of this post, we are going to focus solely on these leukocyte cells.
What are leukocytes?
Leukocytes can be divided into two different groups based on what they look like under a microscope:
- granulocytes – which have tiny granules inside them
- agranulocytes – which do not have tiny granules inside them (simple enough)
This division can broken down further into five major subtypes:
Source: KhanAcademy
And while it would bring me great pleasure to discuss the roles of each of these sub-types, I will spare you that fate and dedicate the rest of this post to the cell on the far left (of the image, not political persuasion).
That cell is a lymphocyte.
What is a lymphocyte?
When your is attacked by a pathogen (a disease causing agent) like the common cold virus, it will elicit what is called an immune reaction. Once inside the body, the presence of the virus will be detected by cells in the immune system and given that the virus will be clearly determined to be ‘not self’ (or not part of your body), an immune response will be initiated.
The cells that carry out the immune response are the lymphocytes.

That big cell in the middle is a lymphocyte. Source: ASH
There are basically three types of lymphocytes:
- B cells
- T cells
- Natural killer cells (Sounds cool right?)
B-cells, T-cells and Natural killer cells are highly specialised blood cells that defend our body when things go wrong (which they inevitably do).
B-cells are bone marrow-derived cells which produce antibodies that are used to attack invading pathogens (such as viruses). The ‘B’ actually comes from the name of the place they were discovered, the Bursa of Fabricus. The Bursa is an organ only found in birds.
Source: Askabiologist
B-cells do not actually kill pathogens, they just spend their short life producing antibodies which trap and neutralise them. The killing of pathogens is left to T-cells and natural killer cells. Thymus-derived T-cells kill cells can be identified by the presence of a T-receptor on surface of the cell. This T-receptor identifies antigens (a molecule capable of inducing an immune response) and binds to them. There are three major classes of T-cells that play specific roles in the destruction of antigens. They are:
- Cytotoxic T-cells – which directly terminate cells containing antigens by binding to them and killing them.
- Helper T-cells – which facilitate the production of antibodies by B-cells and also produce substances that activate other T-cells.
- Regulatory T-cells – which actively suppress the response of B-cells and other T-cells to antigens.
The most basic way to think of B-cells and T-cells is that B-cells take care of pathogens outside of cells, while T-cells deal with pathogens that have already infected cells.
Source: Glogster
Natural killer cells are activated in response to a family of chemical messengers (or cytokines) called interferons. Activated natural killer cells go on to release cytotoxic (or cell-killing) granules which then destroy an affected cells (eg. tumor or viral infected cell).
How is a Natural killer cell different from a T-cell?
While natural killer cells act in a very similar manner to cytotoxic T-cells, they are not T-cells.
- They do not have T cell receptors or trigger antibody production.
- T-cells respond to very specific antigens, the response of natural killer cells to an antigen is nonspecific.
- They are capable of distinguishing infected or cancerous cells from normal cells, and can simply attach to any cell that they bump into.
Now I appreciate that this has been a longer than usual intro/biology lesson, but it is important to know some of these blood cell basics and it will help in understanding the research report we are going to review today.
And what is the report we’re discussing today?
This one:
Title: Th17 Lymphocytes Induce Neuronal Cell Death in a Human iPSC-Based Model of Parkinson’s Disease.
Authors: Sommer A, Maxreiter F, Krach F, Fadler T, Grosch J, Maroni M, Graef D, Eberhardt E, Riemenschneider MJ, Yeo GW, Kohl Z, Xiang W, Gage FH, Winkler J, Prots I, Winner B.
Journal: Cell Stem Cell. 2018 Jul 5;23(1):123-131.
PMID: 29979986
In this study, the researchers wanted to look at the role of T-cells in the context of Parkinson’s.
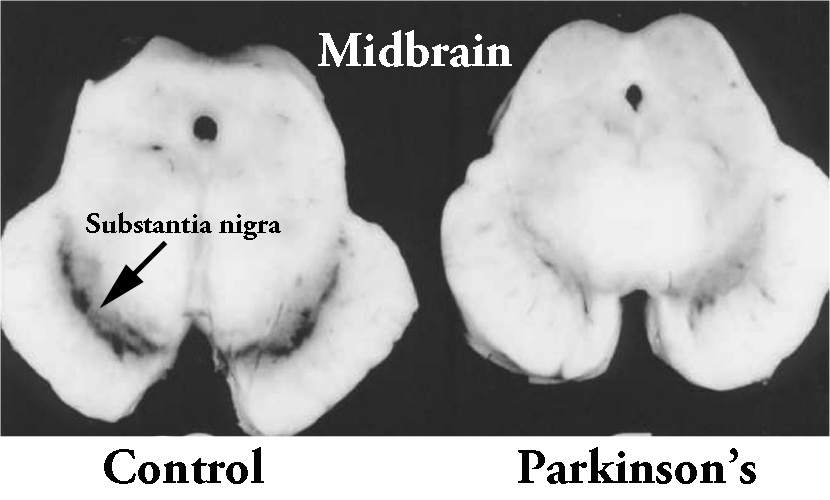
They began their analysis by looking at sections of postmortem brain tissue from a region of the brain that is badly affected by Parkinson’s: the substantia nigra in the midbrain. The area of the brain where the dopamine-producing cells reside. These cells are particularly affected in Parkinson’s, with approximately 50% of them being lost by the time of diagnosis.
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinson’s brain (right). Source: Memorangapp
The investigators found that T-cells were located close to dopamine neurons in sections of brain tissue from Parkinsonian brains, but they were only observed in the blood vessels in control subjects.
Next the investigators analysed blood from 10 people with Parkinson’s and 10 control subjects and they noted a significant increase in the frequency of IL-17-producing Helper T-cells in the people with Parkinson’s.
One small detail here: Helper T-cells can be divided into subsets:
- Interferon producing Helper T-cells – these are called type 1 (or Th1) cells
- IL-4-producing Helper T-cells – these are called type 2 (or Th2) cells
- IL-17-producing Helper T-cells – these are called Th17 cells
The researchers only saw a significant difference in the frequency of IL-17-producing Helper T-cells (Th17) in the people with Parkinson’s (compared to controls), they did not see any difference in frequencies of Th1 or Th2 cells. And the amount of L-DOPA treatment did not appear to affect this result.
What is IL-17?
Interleukin 17 (or IL-17) is a pro-inflammatory cytokine.
What is a cytokine?
Cytokines (from the Greek: kýtos meaning ‘container, body, cell’; and kī́nēsis meaning ‘movement’) are small proteins that are secreted by certain cells in the body and they have an effect on other cells. Cytokines are a method of communication for cells.

How cytokines work. Source: SBS
In the case of Interleukin 17 (or IL-17), the signal that it passes on to the cells it attaches is one of inflammation.
Ok, so what did the researchers do next?
Next the investigators collected skin cells (or fibroblasts) from a biopsy and T-cells from blood samples from people with idiopathic (or spontaneous) Parkinson’s and from healthy age-matched control subjects.
They researchers took the skin cells and turned them into iPS cells.
What are iPS cells?
We live in an age where skin cells (or fibroblasts) can be converted into neurons via a trick of molecular biology. Those skin cells became induced pluripotent stem (or iPS) cells (which you can read about by clicking here). And these iPS cells can be subsequently encouraged to become any cell that you want – such as a neuron (or brain cell) – which gives rise to the possibility of more personalised approaches to research and medicine.
Making iPS cells. Source: learn.genetics
When the investigators grew dopamine neurons from the iPS cells they had collected from each person (each set of iPS cells was kept separate, specific to each participant), and then they exposed those neurons with the T-cells collected from each person, the researchers observed an increase in cell death in the cell cultures from the cells collected from people with Parkinson’s.
Using a ratio of either one dopamine neurons: one T-cell, or one dopamine neuron: ten T-cells, they reported increased levels of cell death in the PD cultures. By comparison, even when they left the cultures grown for long periods of time (10 days+), they witnessed very little cell death in the control samples.
Wow, that’s really interesting. What did they do next?
Given that their analysis of the blood had revealed an increase in IL-17-producing Helper T-cells in the people with Parkinson’s, the researchers naturally turned their attention to these particular cells.
To determine whether IL-17 production could be causing the T cell-mediated dopamine cell death, cell cultures were treated with either high levels of IL-17 or IL-17 receptor inhibitors (blocking the effect of the receptor). In those studies, the researchers found that high levels of IL-17 increased the levels of cell death observed, while the IL-17 receptor inhibitors rescued the T-cell-mediated neuronal cell death in
the Parkinson’s co-cultures (both T-cells and dopamine neurons).
This finding led the researchers to propose a model of IL-17-induced neuronal cell death following T-cell interaction in Parkinson’s.
Is this the first time IL-17-producing Helper T-cells have been implicated in Parkinson’s?
No, it is not.
T-cells have long been suspected of being involved in the cell death associated with Parkinson’s (Click here and here for examples of research related to this). And IL-17-producing Helper T-cells have been implicated:
Title: Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease.
Authors: Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE.
Journal: J Immunol. 2010 Mar 1;184(5):2261-71.
PMID: 20118279 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers used a neurotoxin-based (MPTP) mouse model of Parkinson’s. When they added high levels of a particular form of the Parkinson’s-associated protein alpha synuclein, they found that the neurodegeneration was exaggerated. Via a series of experiment, the investigators found that the increased degenerative cell loss involved, in significant measure, IL-17-producing Helper T-cells which caused dysregulation in regulatory T-cells.
Remind me again: what is a regulatory T-cell?
So up above, we talked about three types of T-cells (Cytotoxic T-cells, Helper T-cells, and Regulatory T-cells). Regulatory T-cells (or Treg cells) are the cells that maintain order in the immune system. They do this by enforcing a dominant negative regulation on other immune cells, particularly other T-cells.
Think of T-cells as the inquisitive neighbours curious about and snooping around a local crime scene, and then imagine that Treg cells are the police telling them “nothing to see here, move along”.

Tregs maintaining order. Source: Keywordsuggestions
We have previously discussed the results of a clinical trial in Nebraska involving the regulation of Treg cells that had interesting results (Click here to read that post).
The results of this previous animal model study suggested that IL-17-producing Helper T-cells were interfering with the ability of regulatory T-cells to do their job, which caused increased cell loss.
Interesting. So summing: What does it all mean?
No, hang on.
Sorry, but we are not finished yet.
One of the most interesting aspects of the new study looking at T-cells from people with Parkinson’s is that one of the IL-17 inhibitors that they used in the study was Secukinumab.
What is Secukinumab?
Secukinumab (also known as Cosentyx) is a human monoclonal antibody that binds to and inhibits the actions of IL-17. It is marketed by the pharmaceutical company Novartis, and it is used for the treatment of psoriasis, ankylosing spondylitis, and psoriatic arthritis.
Source: Wikidoc
Approved by the FDA in January 2015 to treat adults with psoriasis – a common immune-mediated skin condition that has a curious association with Parkinson’s (Click here to read more about that) – Secukinumab has subsequently been approved it to treat adults with arthritis-related conditions (ankylosing spondylitis and psoriatic arthritis in January 2016) and moderate-to-severe scalp psoriasis (February 2018).
So where can I get me some of that Secukinumab stuff?
There are two components to my usual ‘rain on the parade’ warning today:
- Secukinumab is a very powerful immune suppressor. It is the treatment for psoriasis that is used after everything else has failed. And it comes with some nasty side effects (for example, more than 10% of people on this treatment experience upper respiratory tract infections). People with inflammatory bowel disease issues are recommended to avoid it (Click here to read more about this).
- There is some conflicting data regarding the role of IL-17-producing Helper T-cells.
For example, this very recently published study:
Title: Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients
Authors: Kustrimovic N, Comi C, Magistrelli L, Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F, Minafra B, Riboldazzi G, Sturchio A, Mauri M, Bono G, Marino F, Cosentino M.
Journal: J Neuroinflammation. 2018 Jul 12;15(1):205.
PMID: 30001736 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers collected blood from 47 healthy control participants and 82 people with Parkinson’s. The PD participants included 26 subjects who had never been treated with any anti-Parkinson’s drugs (such as Levodopa) before enrollment, and 56 subjects already being treated with anti-Parkinson’s drugs.
The results of this study suggest that people with Parkinson’s have reduced levels of circulating T-cells, due to reduced levels of Th2, Th17, and Treg cells.
The researchers found that the production of pro-inflammatory messengers (like interferon-γ and tumor necrosis factor-α) by T-cells were increased in the blood of people with Parkinson’s, and this was maintained in the presence of the mediating Treg. They also reported that this Th1-biased effect occurs in both the drug-naïve and L-DOPA treated groups with Parkinson’s, suggesting that current anti-Parkinson’s treatments do not affect the peripheral immune system.
And this reduction in levels of IL-17-producing Helper T-cells has been reported by other (Click here to read more about this). These results would indicate that using secukinumab may have no effect (or worse: weaken the immune system further).
Thus, given this contrasting data, the only conclusion that can be drawn at the moment is that a lot more research is required before we start targeting specific populations of T-cells.
So what does it all mean?
My five year old was very impressed with my black board presentation, and at the end of it she surprised me by asking the very insightful question “Papa, is Parkinson’s an autoimmune condition?” (I swear she get her brains from her mother!).
It is a question that many Parkinson’s researchers have been asking for some time, as there are certainly associations between Parkinson’s and other autoimmune conditions (Click here, here, and here to read more about this – and click here to read a previous SoPD post on this topic).
What is very clear is that the immune system is involved with Parkinson’s, and better regulation of that system may provide us with a method of slowing down the progression of Parkinson’s (by slowing the clearance of sick or dysfunctional dopamine neurons).
Such an approach may not really address the driving cause of neurodegeneration in Parkinson’s (what is actually making the cells sick), but it could certainly offer us another component of an ever increasing arsenal of therapies in development for treating this condition.
So – as I said to my 5 year old little monster – “Watch this space”.
The banner for today’s post was sourced from healthhearty.














Simon, thank you for another excellent and very informative post. I am with your 5 year old and I think that the multitude of neurological disorders we combine under the label of PD has strong connection to autoimmune dysfunction.
The fact that there are some contradictory data on IL17 could be due to the fact that many cytokines have dual function promoting inflammation and reducing it. It would be interesting to conduct a meta study on the effect Cosentyx has on PD: enough people with psoriasis take it where a statistically significant sub-population of PwP could be found among them. Actually it would be interesting to do the same for other monoclonal antibody treatments to see if any of them affect PD. Alternatively Prothena PD treatment that targets alpha SN I wonder if it results in reduced levels of IL17 or other cytokines. Another interesting question: Was there any change in PD progression and symptoms in people who underwent successful bone marrow transplant for cancer treatment and who prior to the procedure had PD?
Thank you,
Felix
LikeLike