|
Aspirin is one of the oldest drugs in medical use today. Recently researchers noticed something interesting about ‘low doses’ of aspirin that could have implications for Parkinson’s: It raises the amount of dopamine in the brain Specifically, low doses of aspirin triggers an increase in the levels of an enzyme called tyrosine hydroxylase, which is involved in the production of chemical dopamine. Given that levels of dopamine are severely reduced in the brain of a person with Parkinson’s, this new result is kind of interesting. In today’s post, we will have a look at what aspirin and tyrosine hydroxylase are, what the new research results report, and what this could mean for the Parkinson’s community.
|
Source: Ancientpages
The Ebers Papyrus (also known as the also known as Papyrus Ebers) is considered one of the most oldest medicinal “encyclopedias”.
It outlines 700 Egyptian medicinal formulas and remedies dating back to circa 1550 BC. We know nothing about who wrote the document (even the source of the papyrus is unknown – it may have been found with a mummy in the El-Assasif district of the Theban necropolis).
One thing is clear though: the people who wrote it were a very far sighted bunch.
Interestingly, the papyrus mentions use of Willow bark and Myrtle to treat fever and pain. Both of these plants are rich in Salicylic acid.
What is Salicylic acid?
It is an active precursor (or metabolite) of acetylsalicylic acid – which is also known as ‘Aspirin’.
Source: Labmuffin
What exactly is aspirin?
Aspirin is a very common medication that is used in the treatment of pain, fever, or inflammation. Even in its modern form, it is one of the oldest drugs in currently medical use.
Source: ox.ac.uk
The name “aspirin” comes from Spiraea, a biological family of shrubs, which all contain increased levels of salicylic acid (salicylic acid can also be found in jasmine, beans, peas, clover, and certain grasses).
And it wasn’t just the Egyptians who recognised the effect of salicylic acid, many ancient societies used plants containing it. For example, Hippocrates – the Greek physician (460 to 377 B.C.) – wrote about the use of tea made from willow leaves and bark to relieve pain and fevers.
It took thousands of years, however, before people actually isolated salicylic acid, and even then it was sparsely used due to its unpleasant taste and tendency to damage the stomach. It was not until 1897, that scientists at the pharmaceutical company Friedrich Bayer & Co. (now just ‘Bayer’) found a way to create a stable form of the drug that was easier and more pleasant to take.
Two years later (on March 6th, 1899), Bayer patented “Aspirin” and the rest is history.
Source: dingeengoete
In addition to alleviating pain, Aspirin is given shortly after a heart attack in order to decrease the risk of death. Long-term use of it has been shown to help prevent heart attacks, ischaemic strokes, and blood clots in people at high risk, as well as decreasing the risk of certain types of cancer (particularly colorectal cancer).
And before you jump on the band wagon, over use of Aspirin has been shown to increase the risk of gastrointestinal bleeding. It should not be taken in excess (PLEASE speak with your doctor before considering any change to your treatment regime).
How does aspirin work?
Aspirin suppresses the production of prostaglandins and thromboxanes.
What are prostaglandins and thromboxanes?
They are two proteins in the body that cause constriction (or dilation) in vascular smooth muscle cells, AND they cause aggregation of platelets (the fragments floating around in your blood that help to stop bleeding).
And aspirin achieves that inhibition by the irreversible inactivation of the cyclooxygenase-1 (or COX-1). And COX-1 is an enzyme required for the production of prostaglandins and thromboxanes.
The mechanism of action for aspirin. Source: doctorshangout
But how does aspirin reduce pain?
To save time, I’ll let Bayer explain via this video:
Interesting. So why is aspirin interesting for a Parkinson’s blog?
This week, an interesting report was published:
Title: Low-Dose Aspirin Upregulates Tyrosine Hydroxylase and Increases Dopamine Production in Dopaminergic Neurons: Implications for Parkinson’s Disease.
Authors: Rangasamy SB, Dasarathi S, Pahan P, Jana M, Pahan K.
Journal: J Neuroimmune Pharmacol. 2018 Sep 5.
PMID: 30187283
In this study, the researchers tested different doses of aspirin (for 2 hours) on dopamine neurons being grown in culture, and they found that low doses of aspirin increased levels of tyrosine hydroxylase RNA (the instructions for making protein) and tyrosine hydroxylase protein.
What is tyrosine hydroxylase?
Tyrosine hydroxylase (or simply TH) is the enzyme involved in the production of the chemical dopamine. Specifically, TH converts a protein called Tyrosine (which is absorbed into brain cells from the blood) into L-dopa. L-dopa is naturally produced in the brain and it is turned into dopamine by another enzyme (called Aromatic L-amino acid decarboxylase or AADC). L-dopa is also the basis of many treatments for Parkinson’s (such as Levodopa – commercial versions of Levodopa include ‘Sinemet’).
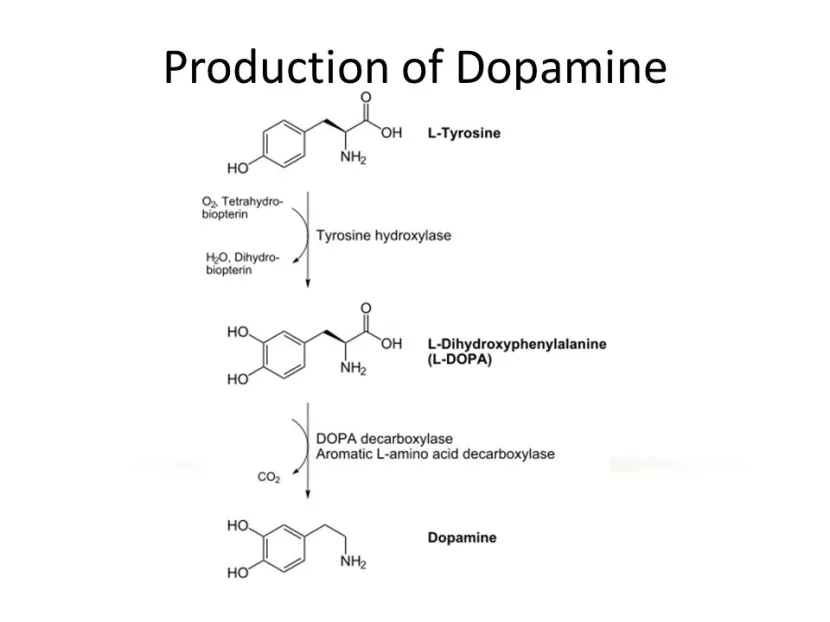
The production of dopamine. Source: Slideplayer
Ok, so TH helps to produce dopamine, but why is dopamine important?
Dopamine is a chemical in the brain that helps us to move freely. Without it, our movements become inhibited – as in the case of Parkinson’s.
Parkinson’s is a condition of inhibition – people want to move but they can’t, because their movement becomes inhibited. The loss of dopamine plays an important role increased level of inhibition.
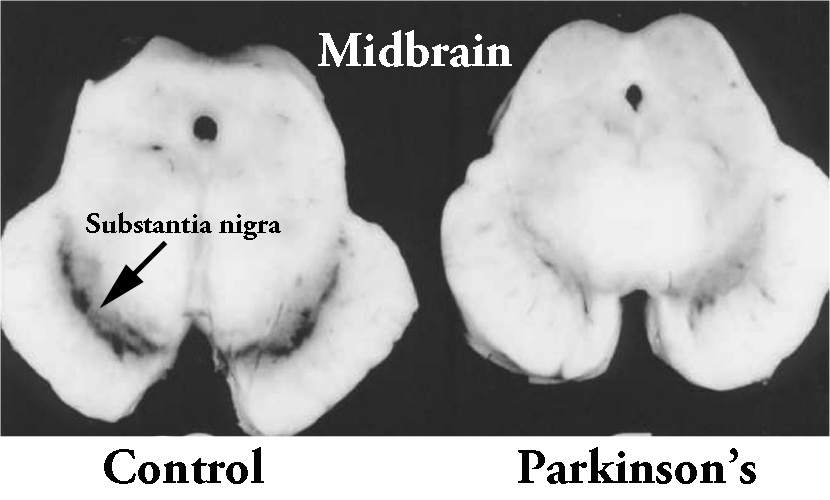
The majority of the dopamine produced in your brain is made in a region called the substantia nigra, deep inside you brain. These dopamine producing cells also generate another chemical called neuromelanin, which has a dark colouration to it – making it visible to the naked eye. As you can see in the image below, the substantia nigra region is easy to see on the section of healthy control brain on the left, but less visible on the section of brain from a person who passed away with Parkinson’s.
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinson’s brain (right). Source: Memorangapp
The dopamine neurons of the substantia nigra release their dopamine in different areas of the brain. The primary regions of that release are areas of the brain called the putamen and the caudate nucleus. The dopamine neurons of the substantia nigra have long branches/projections (called axons) that extend a across the brain to the putamen and caudate nucleus, so that dopamine can be released there.
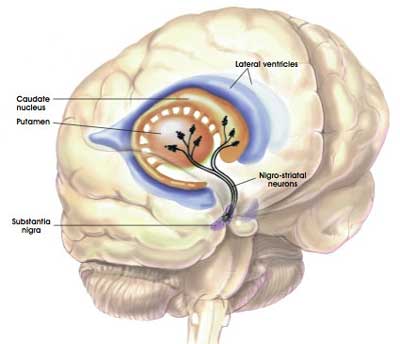
The projections of the substantia nigra dopamine neurons. Source: MyBrainNotes
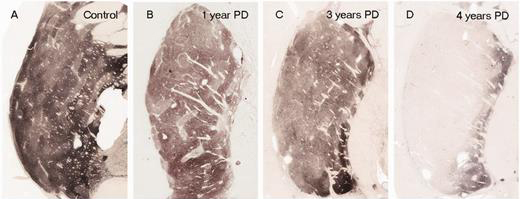
In Parkinson’s, these ‘axon’ extensions that project to the putamen and caudate nucleus gradually disappear as the dopamine neurons of the substantia nigra are lost. When one looks at brain sections of the putamen after the axons have been labelled with a dark staining technique (such as the image below), this reduction in axons is very apparent over time, especially when compared to a healthy control brain (PLEASE NOTE: that the progression of fibre loss in the putamen can vary from person to person and the image provided here is presented as an example).
The putamen in Parkinson’s (across time). Source: Brain
But why does the loss of dopamine cause movement to become inhibited?
Movement is largely controlled by the activity in a specific group of brain regions, collectively known as the ‘Basal ganglia‘. As you can see in the image below, the putamen and caudate nucleus are key components of the basal ganglia:

The basal ganglia structures (blue) in the human brain. Source: iKnowledge
But while the basal ganglia controls movement, it is not the starting point for the movement process.
The prefrontal cortex is where we do most of our ‘thinking’. It is the part of the brain that makes decisions with regards to many of our actions, particularly voluntary movement. The prefrontal cortex (the green area in the image below) is involved in what we call ‘executive functions’.

Areas of the cortex. Source: Rasmussenanders
Now the prefrontal cortex might come up with an idea (for example, ‘the left hand should start to play the piano’). It will communicate this idea with the premotor cortex (the blue area in the image above), and together they will send a very excited signal down into the basal ganglia for it to be considered.
Now in this scenario it might help to think of the cortex as a bunch of hyperactive, completely-out-of-control toddlers, and the basal ganglia as the parental teacher in a class room setting. All of the toddlers are making demands/proposals and sending mixed messages, and it is for the inhibiting teacher (the basal ganglia) to gain control and decide which message is the best.
The message coming from the cortex. Source: Getty
So the basal ganglia receives multiple signals from the cortex, processes that information before sending a signal on to another important participant in the regulation of movement: the thalamus.

A brain scan illustrating the location of the thalamus in the human brain. Source: Wikipedia
The thalamus is a structure deep inside the brain that acts like the central control unit of the brain. Everything coming into the brain from the spinal cord, passes through the thalamus, and everything leaving the brain, passes through the thalamus. It is aware of most everything that is going on and it plays an important role in the regulation of movement. If the cortex is the toddler and the basal ganglia is the teacher, then the thalamus is the extremely strict head teacher.
Now to complicate things for you further, the processing of movement in the basal ganglia involves a direct pathway and an indirect pathway. In the simplest terms, the direct pathway encourages movement, while the indirect pathway does the opposite: inhibits it.

Source: Studyblue
The thalamus will receive signals from the two pathways and then decide – based on those signals – whether to send an excitatory or inhibitory message to the primary motor cortex, telling it what to do or not to do (‘tell the muscles to play the piano’ or ‘don’t start playing the piano’, respectively).
The primary motor cortex is the red stripe in the image below.

The primary motor cortex then sends this structured message down the spinal cord (via the corticospinal pathway) and all going well the muscles in the left arm will do as instructed.

Source: adapted from Pinterest
Ok, but what happens in Parkinson’s?
In Parkinson’s, the motor features (slowness of movement and resting tremor) are associated with a breakdown in the processing of those direct and an indirect pathways. This breakdown results in a stronger signal coming from the indirect pathway – thus inhibiting/slowing movement.
This situation results from the loss of dopamine in the brain.

Excitatory signals (green) and inhibitory signals (red) in the basal ganglia, in both a normal brain and one with Parkinson’s. Source: Animal Physiology 3rd Edition
Under normal circumstances, dopamine neurons release dopamine in the basal ganglia that helps to mediate the local environment. It acts as a kind of lubricant for movement, the oil in the machine if you like. It helps to reduce the inhibitory bias of the basal ganglia (it makes the teacher in our classroom analogy more relaxed and less domineering).
Thus, with the loss of dopamine neurons in Parkinson’s, there is an increased amount of inhibitory activity coming out of the indirect pathway (and the teacher becomes more strict and inhibiting).
And as a result, the thalamus is kept in an overly inhibited state. With the thalamus subdued, the signal to the motor cortex is unable to work properly. And this is the reason why people with Parkinson’s have trouble initiating movement.

Source: BJP
As a result of this situation, we supplement the missing dopamine, by treating people with Parkinson’s with L-dopa.
I see. And the new report suggests that aspirin could be used instead of L-dopa?
No.
The new report suggests that low doses of aspirin can increase the level of tyrosine hydroxylase. And this in turn, the researchers found, resulted in increased levels of dopamine. It is important to appreciate that this effect occurred only in a narrow range of low doses – as the doses of aspirin increased, the effect was lost.
But the researchers found that oral administration of low dose aspirin increased the levels of TH in the substantia nigra and raised levels of dopamine being released in the striatum of not only normal mice, but also aged genetically engineered mice.
Source: Pinterest
The genetically engineered mice carried a variation in their DNA called A53T.
What is A53T?
There is a region of your DNA that provides the instructions for making alpha syncuclein. That region of DNA is called SNCA. And there are several genetic variations (or mutations) inside of SNCA that are associated with an increased risk of developing Parkinson’s.
A53T is the name of one of those genetic variations.
As you can see in the image below, A53T lies in the red (Amphipathic) region of SNCA along with several other genetic variants, such as A30P and E46K:
The researchers found that aged mice carrying this A53T genetic variation improved in their locomotor activities after treatment with low dose aspirin.
Has aspirin ever been associated with Parkinson’s before?
Recently there has been a manuscript released on BioRxiv that reports a study which found a potential association between Parkinson’s and aspirin:

Title: The Parkinson’s Phenome: Traits Associated with Parkinson’s Disease in a Large and Deeply Phenotyped Cohort
Authors: Heilbron K, Noyce A, Fontanillas P, Alipanahi B, The 23andMe Research Team, Nalls M, Cannon P
Journal: bioRxiv preprint first posted online 28th February, 2018
PMID: N/A (This article is OPEN ACCESS if you would like to read it)
We have previously discussed this manuscript at length (Click here to read that post), but something we did not discuss in that post was that the researchers found that taking aspirin daily ‘may’ be associated with a reduced incidence of Parkinson’s (this finding needs to be confirmed in a larger study though, as a previous study by some of the same researchers found that aspirin was not associated with Parkinson’s – click here to read that report).
So what does it all mean?
Recently researchers have found that low doses of the pain reliever, aspirin, can increase levels of dopamine in the brains of mice. This finding is interesting because it demonstrates a novel mechanism by which this commonly used drug works. But it is also particularly intriguing for the Parkinson’s community.
It not only highlights a new area of research that could help in the production of novel future therapies, based around this mechanism, but it also raises awareness about a possible confounding factor in the use of L-dopa-based therapies. Perhaps if the results can be independently replicated, a cautionary note should be added to the boxes/bottles of aspirin regarding its use alongside L-dopa?
As I say, the result needs to be replicated, but the finding is interesting.
Addendum: 17/09/2018 – an additional cautionary note:
Two reports were published in the New England Journal of Medicine today, presenting results from the Aspirin in Reducing Events in the Elderly (ASPREE) study.
The ASPREE study was designed to determine whether taking a daily low-dose aspirin would extend the length of a disability-free life in healthy participants aged 65 years and above. 19,114 people (>65 years of age) from Australia and the US were enrolled in this study, 9525 were assigned to receive aspirin and 9589 to receive placebo. The participants took low dose aspirin for (on average) 4 1/2 years (Click here to read more about the details of this study)
The results of the study found no health benefits from taking low dose aspirin for healthy individuals over the age of 70. In addition, trial participants taking daily aspirin were more likely to suffer from serious bleeding (3.8 per cent of the group), than the placebo group (2.8 per cent) (Click here and here to read those reports and click here to read the editorial).
So again, please consult your doctor before contemplating any changes to your treatment regime.
EDITORIAL NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. PLEASE speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Healthline










I find your posts so interesting to read. The referencing is excellent. Thank You!
LikeLike
Hi Anthony,
Thanks for your comment – glad you like the material.
Kind regards,
Simon
LikeLike
My first thought is that aspirin can lower blood pressure. Many people with Parkinson’s suffer from low blood pressure. My concern is that fall risk could increase with further drops in blood pressure.
LikeLike
Hi Lisa,
Thanks for your interesting comment. You are correct and I fully agree. But this post was never intended as an alternative method of increasing dopamine levels (which would be tricky given the narrow window of doses observed in this study). It was only written because the new research results were so interesting. It would also be interesting to analyse whether people with PD who take aspirin have different disease course compared to those who do not (based on this possible dopamine effect, and also the blood pressure issue).
Kind regards,
Simon
LikeLike
I am desperately searching for relief fromRLS restless legs syndrome. If aspirin increase dopamine production this may a a window of opportunity. As of what I have read on this site, as
Iron is not a viable option.
LikeLike
I had insomnia for years with RLS I literally jumped around the bed all night and while watching tv. I tried magnesium it didn’t work. My GP prescribed 1 Pramipexole – it worked. However this was a year before I was diagnosed with Parkinson’s. I now have Pramipexole 6 a day. The only good thing is that I no longer have RLS at night! I am in the UK
LikeLike