|
Not a week goes by without some new peice of research suggesting yet another biological mechanism that could be useful in slowing or stopping Parkinson’s. This week researchers in Chicago reported that pharmacologically inhibiting a specific enzyme – farnesyltransferase – may represent a novel means of boosting waste disposal and helping stressed cells to survive. A number of farnesyltransferase inhibitors are being developed for cancer, and there is the possibility of repurposing some of them for Parkinson’s. In today’s post, we will discuss what farnesyltransferase is and does, what the new research report found, and we will consider whether inhibition of this biological pathway is do-able for Parkinson’s.
|
 Source: Knowledgepathinc
Source: Knowledgepathinc
I am in the midst of preparing the “end of year review” and “road ahead” posts for 2019/2020 (they take a while to pull together). But it is already extremely apparent that we have an incredible amount of preclinical data piling up,…. and a serious bottleneck at the transition to clinical testing.
It is actually rather disturbing.
Previously this was a concern, but going forward – as more and more novel preclinical work continues to pile up – one can foresee that it is going to be a serious problem.
But there is just SOOOO much preclinical data on Parkinson’s coming out at the moment. Every single week, there is a new method/molecular pathway proposed for attacking the condition.
A good example of this frenetic pace of preclinical research is a recent report from researchers in Chicago, who discovered that a farnesyltransferase inhibitor could be beneficial in Parkinson’s.
Farne…syl… what?
Quite often in biology, for a protein to perform a specific function it must be acted on by another protein, which may change the configuration of the protein or add/subtract something to activate it.
Farnesyltransferase is an example of this.
It is an enzyme that attaches farnesyl groups to other proteins.
 Farnesyltransferas (ooh, rotating image). Source: Proteopedia
Farnesyltransferas (ooh, rotating image). Source: Proteopedia
The addition of farnesyl groups is essential for those proteins to perform their functions. If farnesyltransferase does not attach farnesyl groups to certain proteins, those proteins will not be able to do their jobs.
The cancer-associated protein Ras is a good example. Farnesylpyrophosphate is necessary for Ras to attach to the cell membrane – see image below (click here to read more about this). If it does not attach to the cell membrane, Ras is not able to carry out its function which involves transfering signals from receptors on the membrane to the interior of the cell. Thus, Ras needs to interact with farnesyltransferase inorder to do its job.
 Farnesyltransferase and Ras. Source: iiar
Farnesyltransferase and Ras. Source: iiar
A farnesyltransferase inhibitor (sometime called an FTI) is a compound that prevents the farnesyltransferase enzyme from attaching farnesyl groups to other proteins, and in doing so blocks the function of that protein.
Ras is overactive in many different types of cancers, and by treating people who have those cancers with farnesyltransferase inhibitors, we are able to help weaken the cancer cells ability to grow.
In other cases, the addition of a farnesyl group will inhibit a biological process, and help to maintain some kind of equilibrium.
And this is the case with what the researchers in Chicago found.
Interesting. But what did the researchers from Chicago discover specifically?
Here is their report:
 Title: Stress-Induced Cellular Clearance Is Mediated by the SNARE Protein ykt6 and Disrupted by α-Synuclein
Title: Stress-Induced Cellular Clearance Is Mediated by the SNARE Protein ykt6 and Disrupted by α-Synuclein
Authors: Cuddy LK, Wani WY, Morella ML, Pitcairn C, Tsutsumi K, Fredriksen K, Justman CJ, Grammatopoulos TN, Belur NR, Zunke F, Subramanian A, Affaneh A, Lansbury PT Jr, Mazzulli JR
Journal: Neuron, 2019 Oct 9. pii: S0896-6273(19)30774-3.
PMID: 31648898
In this study, the researchers were interested in what happens to SNARE proteins when cells are stressed in the context of Parkinson’s.
What are SNARE protein?
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (or SNARE; sometimes known as “SNAP REceptor”) is a large structure of proteins (often more than 60 in mammalian cells), that mediate the fusing of vesicles inside of cells.
What are vesicles?
Vesicles are small bags of ingredients inside of cells that are surrounded by a lipid membrane – similiar to the membrane that surrounds the entire cell:
 A vesicle. Source: Wikipedia
A vesicle. Source: Wikipedia
These vesicles will contain all kinds of contents, from food to be broken down to the mixtures of enzymes for breaking down that material. The former – vesicles of food – are called vacuole, while the latter – the bags of enzymes – are referred to as lysosomes:
So SNARE proteins are involved in those bags (or vesicles) fusing?
Yes, SNAREs help the vesicles come together and share their contents. It is a critical function in cellular biology. If something goes wrong in this process, there is a disruption of ‘lysosomal activity’ (lysosomes full of digestive enzymes are unable to do their jobs) and waste starts to build up, cause stress inside the cell.
The researchers from Chicago wanted to better understand what happens to SNARE proteins when cells are stressed in Parkinson’s.
So they started by growing cells that carry a genetic mutation in the alpha synuclein gene (A53T), which results in more alpha synuclein protein accumulation (or aggregation). The accumulation of alpha synuclein inside of certain cells is one of the hallmarks of Parkinson’s (click here to learn more about alpha synuclein).
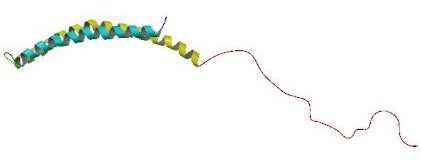 Alpha synuclein. Source: Wikipedia
Alpha synuclein. Source: Wikipedia
They began by assessing lysosomal function in the presence of accumulating alpha synuclein cells over time in culture. This investigation revealed an age-dependent decline in lysosomal activity (particularly between days 75 and 130 in culture), and that this reduction occured subsequent to alpha synuclein protein aggregation.
They next determined that a critical SNARE protein called Ykt6 not only interacts with alpha synuclein, but its activity is disrupted by that interaction – which results in less SNARE activity. Of interest here is that the researchers presented data suggesting that Ykt6 levels are reduced in the postmortem Parkinson’s brain.
Hang on a second. What exactly is Ykt6? And what does it do? It sounds like a Star Wars character.
Ykt6 is a protein that exists floating around inside of cells, primarily in an inhibited state. When the cell becomes stressed, Ykt6 becomes unihibited, opens up, and starts switching on its SNARE activity to enhance lysosomal activity.
The researchers in this new study found that alpha synuclein actually traps Ykt6 in its closed, inactive conformation. And this entrapment reduces the ability of Ykt6 to help stressed cells (by enhancing lysosomal activity). The investigators were quick to realiase that is situation could a.) cause the cell further stress, and b.) represent a novel therapeutic target for Parkinson’s.
Curiously, this is not the first time Ykt6 has been implicated in alpha synuclein associated-toxicity – one previous study suggested that high levels of Ykt6 was neuroprotective against alpha synuclein toxicity (Click here and here to read two previous studies linking this protein to Parkinson’s).
Interesting. So what did the researchers do next?
The investigators conducted some studies looking at what happens when they reduce Ykt6 levels in cells and they found that levels of some of the lysosomal enzymes dropped, reducing lysosomal activity. Of particular interest, they found that by reducing levels of Ykt6, there was a subsequent lowering of glucocerebrosidase levels. And when they increased Ykt6 levels, glucocerebrosidase levels increased.
Cool…. but what is the heck is glucocerebrosidase?
Glucocerebrosidase (also known as GCase) is a lysosomal enzyme that is closely associated with Parkinson’s. Genetic variations in the section of DNA that produces glucocerebrosidase (known as the GBA gene) are some of the most common genetic risk factors for Parkinson’s (Click here to read a previous SoPD post on GCase and GBA-associated Parkinson’s).
 Source: Prevail
Source: Prevail
Ok, so low Ykt6 levels = low Parkinson’s-associated GCase levels?
Yes. And this is actually really interesting as it could help to explain why some people with Parkinson’s, but no genetic variation in their GBA gene have reduced levels of GCase activity (Click here to read more about this).
Got it. What did the researchers do next?
So this is where farnesyltransferase inhibition comes into the story. Normally, Ykt6 is maintained in a closed and inactive state by a farnesyl group. When cells become stressed, that farnesyl group is removed and Ykt6 is able to get on with its job.
The researchers started wondering if removing the farnesyl group from Ykt6 might be a method of improving lysosomal activity in the context of alpha synuclein toxicity. Firsty, they demonstrated that a mutant form of the Ykt6 protein which remains open and active continuously, significantly reduces alpha synuclein toxcity. Second, they tested pharmacological inhibition of farnesyltransferase – the protein that adds farnesyl group to Ykt6.
To do this, they tested a drug called LNK-754. This is a farnesyltransferase inhibitor that has previously been clinically tested for cancer (Click here to read more about this). The researchers administered LNK-754 to dopamine neurons in cell culture that produce high levels of alpha synuclein. They found that LNK-754 administration partially restored GCase activity and also reduced levels of toxic alpha synuclein. They also conducted these studies in genetically engineered mice which produce high levels of alpha synuclein and reported the same result.
The researchers concluded their study suggesting that farnesyltransferase inhibition “activates Ykt6 in the brains of mice to stimulate the lysosomal system and reduce pathological alpha synuclein“, which implies “a strong therapeutic potential of this pathway for the treatment of synucleinopathies” (like Parkinson’s).
Is this the first time farnesyltransferase inhibition has shown beneficial properties in models of Parkinson’s?
Actually, no.
Way back in 2009, this report was published:
 Title: Membrane-associated farnesylated UCH-L1 promotes alpha-synuclein neurotoxicity and is a therapeutic target for Parkinson’s disease.
Title: Membrane-associated farnesylated UCH-L1 promotes alpha-synuclein neurotoxicity and is a therapeutic target for Parkinson’s disease.
Authors: Liu Z, Meray RK, Grammatopoulos TN, Fredenburg RA, Cookson MR, Liu Y, Logan T, Lansbury PT Jr.
Journal: Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4635-40.
PMID: 19261853 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to investigate in the role of UCH-L1 in cells.
What is UCH-L1?
Ubiquitin C-terminal hydrolase-L1 (UCH-L1) is a protein that is produced at high levels in neurons. Genetic variations in the section of DNA (or gene) that produces UCH-L1 is associated with an increased risk of developing Parkinson’s (Click here to read more about the genetics of Parkinson’s).
 UCH-L1. Source: Wikipedia
UCH-L1. Source: Wikipedia
There appears to be two forms of the UCH-L1protein: one floating around interacting with other proteins and the other attached to membranes in the cell. And the researchers who conducted this study found that the amount of membrane-attached UCH-L1 in correlated with the level of alpha synuclein in the cell.
They also found that high levels of membrane-attached UCH-L1 exaggerated alpha synuclein toxicity. But they discovered that UCH-L1 is farnesylated in order to bind to membranes, and when it is not farnesylated, there is no accumulation of alpha synuclein. By adding a farnesyltransferase inhibitors to cell cultures of cells producing toxic levels of alpha synuclein, the researchers found that they could prevent the cells from dying.
At the time, the investigators did not know that lysosomal activity was associated with this beneficial effect of farnesyltransferase inhibitors, but they did conclude that “inhibition of UCH-L1 farnesylation may be a therapeutic strategy for slowing the progression of Parkinson’s“.
Is this farnesyltransferase inhibitor effect specific to Parkinson’s?
It does not appear to be.
Farnesyltransferase inhibition has been explored in other neurodegenerative conditions, such as Alzheimer’s. And there is also research suggesting that reduced farnesyltransferase activity may have a protective on the risk of developing Alzheimer’s:
 Title: Farnesyltransferase haplodeficiency reduces neuropathology and rescues cognitive function in a mouse model of Alzheimer disease.
Title: Farnesyltransferase haplodeficiency reduces neuropathology and rescues cognitive function in a mouse model of Alzheimer disease.
Authors: Cheng S, Cao D, Hottman DA, Yuan L, Bergo MO, Li L.
Journal: J Biol Chem. 2013 Dec 13;288(50):35952-60.
PMID: 24136196 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers took an Alzheimer’s mouse model (APPPS1 mice – these mice have been genetically engineered to produce high levels of a mutant form of the amyloid-β precursor protein (one containing the Swedish double mutation) AND a mutant form of presenilin 1 (containing the deletion of exon 9). These mice begin to exhibit the pathological hallmarks of Alzheimer’s – beta amyloid plaques – from approximately six weeks of age.
The investigators cross-bred these animals with mice that produced 50% less farnesyltransferase, and they found that the offspring not only performed better on cognitive/behavioural tests, but also had less inflammation in their brains and less beta amyloid plaque pathology.
And this research has very recently (March 2019) been replicated pharmacologically using farnesyltransferase inhibitors in mouse models of Alzheimer’s:
 Title: A farnesyltransferase inhibitor activates lysosomes and reduces tau pathology in mice with tauopathy.
Title: A farnesyltransferase inhibitor activates lysosomes and reduces tau pathology in mice with tauopathy.
Authors: Hernandez I, Luna G, Rauch JN, Reis SA, Giroux M, Karch CM, Boctor D, Sibih YE, Storm NJ, Diaz A, Kaushik S, Zekanowski C, Kang AA, Hinman CR, Cerovac V, Guzman E, Zhou H, Haggarty SJ, Goate AM, Fisher SK, Cuervo AM, Kosik KS.
Journal: Sci Transl Med. 2019 Mar 27;11(485)
PMID: 30918111
In this study, the investigators found that a farnesyltransferase inhibitor called lonafarnib had beneficial effects when administered to a Tau mouse model of Alzheimer’s.
What is Tau?
Like beta amyloid and alpha synuclein, Tau is a protein that accumulates in the brains of several neurodegenerative conditions, including Parkinson’s (Click here to read a previous SoPD post on the Tau of Parkinson’s).
The researchers used mice that produce high levels of a mutant form of the Tau protein (it carries the the P301L mutation). These mice begin to display the hall marks of neurodegenerative disease around 4-5 months of age.
By treating these mice with lonafarnib, the researchers found that they could not only resecue the behavioral abnormalities associated with these mice and reduce inflammation in their brains, but they could also decrease levels of Tau protein accumulation and brain atrophy. And they found that this effect was associated with increased lysosomal activity (Click here for an interesting write up about this research on the Scientific American website).
Lonafarnib was designed for and has been extensively studied in cancer, and it is being developed by Eiger BioPharmaceuticals as a treatment for hepatitis-D.
Are there any clinical trials of farnesyltransferase inhibitors in Parkinson’s?
Not that I am aware of.
But it is interesting to note that some of the researchers involved in the Parkinson’s research involving farnesyltransferase inhibition work for a company called Lysosomal Therapeutics.
 To date, Lysosomal Therapeutics has focused on a compound called LTI-291, which boosts GCase activity in GBA-associated models of Parkinson’s. Some of the employees of Lysosomal Therapeutics were previously employed by a biotech firm called “Link Medicine Corporation”, which was developing LNK-754. It will be interesting to see if farnesyltransferase inhibition represents a novel direction for the company.
To date, Lysosomal Therapeutics has focused on a compound called LTI-291, which boosts GCase activity in GBA-associated models of Parkinson’s. Some of the employees of Lysosomal Therapeutics were previously employed by a biotech firm called “Link Medicine Corporation”, which was developing LNK-754. It will be interesting to see if farnesyltransferase inhibition represents a novel direction for the company.
Was LNK-754 ever clinically tested in Parkinson’s?
I am not aware of any farnesyltransferase inhibitors being clinically tested in Parkinson’s.
But LNK 754 was tested in 110 individuals with mild Alzheimer’s for 28 days (Click here to read more about this). It’s clinical development appears to have ended there though.
Perhaps a farnesyltransferase inhibitor should be tested in Parkinson’s?
There is an interesting discussion on the Alzforum website regarding this topic (Click here to read it).
The consensus of the discussion appears to be that farnesyltransferase inhibition is not an easy task. Ykt6 is not the only target of farnesyltransferase – it is attaching farnesyl groups to lots of different proteins. Thus, while inhibition of farnesyltransferase may have positive benefits for alpha synuclein-stressed dopamine neurons, it may have detrimental effects elsewhere in the body.
So if the new report can be independently replicated and validated, it appears that we may need to re-think how we can better target Ykt6.
So what does it all mean?
These days it feels like every week a novel therapeutic target is being discovered for Parkinson’s. I appreciate that this does not speed up the process of clinically testing any new therapies, but it is extremely encouraging for the future. And before this week, I for one had never heard of farnesyltransferase inhibitors. Whether this class of drugs can be repurposed for Parkinson’s is currently being debated and there may not be an easy answer given the nature of the function of farnesyltransferase. But it is stimulating to see so much preclinical research proposing novel approaches to tackling Parkinson’s.
Returning to the topic discussed at the top of this post, I am concerned by the bottleneck issues with the transition from preclinical to clinical testing. It is a good problem to have, but we need to start devising solutions to deal with it.
The banner for today’s post was sourced from Manchester




WOW! WOW! WOW! Really a NOVEL-TIME for researchers and public
SO EXITING for the NEAR FUTURE
Thank You! so much Simon
LikeLike
Hi Parkinson.guru,
Glad you liked the post. As you say, interesting times for Parkinson’s research.
Kind regards,
Simon
LikeLiked by 1 person