|
NOTE: The information in today’s post should not be considered an endorsement of PhotoPharmics or the treatment they are proposing. The author of this blog has had no communication with the company. The information in this post is provided because the author has been asked by readers to discuss it. In October 2018, at the annual International Movement Disorders Society meeting in Hong Kong, a small biotech firm called “PhotoPharmic” presented a poster entitled “Double-blind controlled trial of Spectramax™ light therapy for the treatment of Parkinson’s disease patients on stable dopaminergic therapy.” In the poster provided the results of a study in which 45 participants with Parkinson’s were blindly treated with light therapy for 1 hour each evening over the course of 6 months. At the end of the study, the investigators found clinically meaningful improvements in the MDS-UPSDRS rating scale, as well as significant improvements in non-motor measures. In today’s post, we will discuss what light therapy is, what this study found, and look at what PhotoPharmic plan to do next.
|
 The Tasmanian “light bucket” for Parkinson’s. Source: ABC
The Tasmanian “light bucket” for Parkinson’s. Source: ABC
It might come as a bit of a surprise to some readers, but one of my favourite stories of 2019 from the world of Parkinson’s research originates from Tasmania.
It is a tale that involves a group of Australian Parkinson’s advocates who somehow cottened on to a seemingly inconceivable idea (treating oneself with a homemade light bucket). But their project was embraced by the local Tasmanian community which is helping out with the research, for example the Dorset community men’s shed is helping to make the light buckets.
 The Dorset community men’s shed helps to make the buckets. Source: ABC
The Dorset community men’s shed helps to make the buckets. Source: ABC
And whatsmore they have inspired an Australia-wide “proof-of-concept” clinical trial on the topic.
The trial is being conducted by The University of Sydney School of Medicine and Parkinson’s South Australia. There is also a website where you can follow the various activities surrounding the trial – Click here to see the website.
 Designing the helmet for the Sydney clinical trials. Source: ABC
Designing the helmet for the Sydney clinical trials. Source: ABC
And there is already published research coming out of the clinical study:
 Title: “Buckets”: Early Observations on the Use of Red and Infrared Light Helmets in Parkinson’s Disease Patients.
Title: “Buckets”: Early Observations on the Use of Red and Infrared Light Helmets in Parkinson’s Disease Patients.
Authors: Hamilton CL, El Khoury H, Hamilton D, Nicklason F, Mitrofanis J.
Journal: Photobiomodul Photomed Laser Surg. 2019 Oct;37(10):615-622.
PMID: 31536464
Now to be clear, I do fully not understand the biology behind the idea, and it would be easy for me to make fun of this whole situation. But I really do love this story. The ivory towers of industry and academic research may scoff at such a story, but I hope that this study will lead to something new and wonderful (the way Joy Milne’s “smell of Parkinson’s” has opened new areas of research – click here to read a previous SoPD post about that).
The light bucket “photobiomodulation” clinical trial for Parkinson’s is a great story about the DIY attitude, community sharing/helping, curiosity & some serious left field thinking (Click here to read a prominent newspaper story about this).
Photobiomodulation? Are you serious? How on Earth can LIGHT help with Parkinson’s?
I am serious.
And so are quite a few researchers around the world. A good example of this occurred last year.
At the annual International Movement Disorders Society meeting in Hong Kong last year, a small biotech firm called “PhotoPharmic” presented a poster outlining some interesting data.
The poster was entitled “Double-blind controlled trial of Spectramax™ light therapy for the treatment of Parkinson’s disease patients on stable dopaminergic therapy” (Click here to read the press release) and it outlined the results of a clinical trial assessing light therapy in people with Parkinson’s.
The results of this study have not yet been published, but they are available via a PhD thesis which was submitted at Vrije Universiteit Amsterdam in the Netherlands (Click here to read that thesis – Chapter 7 contains the current study).
The trial enrolled 92 participants with Parkinson’s (45 years or older) across 3 research centers in Europe and the U.S (Amsterdam University Medical Centers, Massachusetts General Hospital, Boston, and Aspen Clinical Research, in Utah). 45 participants were randomized to the Spectramax treated groups, while 47 were randomised to the placebo light group.
The treatment group were given the Spectramax Light Therapy Lamp, which is a table-top device emitting blue/green narrow bandwidth LED light (λ = 450 – 570 nm, 950 Lux).
 The Spectramax LT device. Source: PhotoPharmics
The Spectramax LT device. Source: PhotoPharmics
Hang on a second. What does any of that last part mean? What exactly is a “lux”?
Lux is a standardised unit of measurement of light level intensity, which is commonly referred to as “illuminance” or “illumination”. It is a rather odd unit of measure though – 1 lux is equal to the illumination of a one metre square surface that is one metre away from a single candle:
 Source: Archtoolbox
Source: Archtoolbox
Using this measure, direct sunligh is 50,000-100,000 lux, while normal ambient daylight is 10,000-25,000 lux (on a cloudy day for example).
Source: Comsparkelectrical
Ok, but what does “λ = 450 – 570 nm” mean?
Light can also be measured in waves. λ (or lambda) refers to the wave length of light.
 Source: Wikipedia
Source: Wikipedia
The wavelengths of the visible spectrum (what we are able to see) usually fall between 400 nm and 700 nm.
 Source: Giangrandi
Source: Giangrandi
So when the Spectramax device is emitting light at “λ = 450 – 570 nm”, it is typically on the blue light end of the spectrum.
The control group in the PhotoPharmics was also exposed to a Light therapy device, identical in appearance and operation to the Spectramax device, but it emited broad bandwidth polychromatic light (λ = 430 – 780nm, 100 Lux). This level of illumination is significantly less than the 1000 Lux of the treatment group, and the wave length is much broader – covering the entire visible light spectrum.
At an intensity of 100 Lux, the investigators believed no substantial effect on circadian rhythmicity was to be expected based on previous research (Click here to read more about this).
What is circadian rhythmicity?
Mmmm, yeah. We’ll come back to this and discuss it in more depth further below. Let’s firstly look at the PhotoPharmics study.
Right. What did the study involve?
The 6-month study required the participants to be treated for one hour in the evening (at a fixed time between 6 and 10 PM as decided by the participant, but the session had to end at least one hour prior to going to bed – Click here to read more about the details of this trial).
The primary end point of the study (that is the measure by which the study is deemed to be successful or not) was “change in the combined scores of Parts I, II, and III of the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)” from a baseline (start of the study) score to a 6 months.
Critically, the participants were assessed during the ON state (meaning that they had taken their normal Parkinson’s medication at the time of assessment).
What were the results of the study?
So this is the part that has some very serious Parkinson’s researchers around the world scratching their heads: The study found a clinically meaningful improvement in the Spectramax treated group.
The researchers conducting the study reported an 8 point difference in MDS-UPDRS (p=0.07).
The change from baseline to the end of treatment was a 17.7 improvement for the Spectramax treated participants versus a 9.7 change for the control group. Assuming that everything else was equal and that the participants were blind to which treatment they were receiving, this is an intriguing result.
And there’s more…
The Spectramax treated participants also exhibited a significant 5 point difference in the PDQ-39 (this is a 39-item self-report questionnaire, which assesses Parkinson’s-specific health). And a significant difference was observed in the non-motor aspects of Parkinson’s (as assessed by the MDS-UPDRS Part I, p<0.01). On top of this, Spectramax treated participants also displayed an almost statistically significant reduction in daytime sleepiness (as determined by the Epworth Sleepiness Scale).
Interesting. So what is going to happen next with this research?
At the time of the presentation in Hong Kong, PhotoPharmics “noted that larger double-blind studies are required to confirm these results, and that the company plans to conduct additional clinical trials to further investigate light therapy in neurodegenerative diseases” (Source).
 Source: UPMP
Source: UPMP
But this statement is a bit confused considering earlier actions by the company.
You see, in July 2018 (before the Hong Kong presentations), PhotoPharmics announced that it had submitted its US FDA application for market authorization, via the De Novo pathway. The De Novo pathway is available to organizations that can show low to moderate risk for their medical device, and where no predicate device has been cleared by the FDA. At the time of that particular announcement, the company was “optimistic about market authorization in the coming months”.
12 months+ later I have not found any updates regarding this process.
Based on the known data (taken from the thesis mentioned above), I think it would be useful and prudent to see a replication/validate of the study in a much larger cohort of people with Parkinson’s. It is apparent that some people responded more than others to the Spectramax therapy and it would be useful to determine who those individuals may be (a novel subset of PD that could tell us something about the condition?).
If PhotoPharmics does get approved, however, Spectramax specific bandwidth phototherapy “could be the first treatment for Parkinson’s which offers symptomatic relief without significant side effects” (Source).
Has there ever been any other clinical studies suggesting beneficial effects with light therapy?
Yes, there has.
And there have been several studies exploring light therapy for Parkinson’s:
 Title: Bright light therapy in Parkinson’s disease: a pilot study.
Title: Bright light therapy in Parkinson’s disease: a pilot study.
Authors: Paus S, Schmitz-Hübsch T, Wüllner U, Vogel A, Klockgether T, Abele M.
Journal: Mov Disord. 2007 Jul 30;22(10):1495-8.
PMID: 17516492
In this study, the researchers examined the effects of 30 minutes of bright light therapy per day (for 15 days) on motor symptoms, depression, and sleep in 36 inidividuals with Parkinson’s. It was a randomised, placebo-controlled double-blind study that evaluated illuminance of 7500 lux in the active treatment group and 950 lux in the placebo group. The treatment was administered every morning (1 hour after awakening, but not later than 9 am), with the head at an approximate distance of 20 cm from the device in the active treatment group, and 100 cm in the placebo group.
The investigators found that the treatment led to significant improvements in different components of the UPDRS rating assessment. There was a significant effect reported in Part I (which assesses mood, etc; P<0.01), Part II (measuring activities of daily living; P<0.01), and IV (which looks at complications of therapy; P<0.01). Bright light therapy also improved depression scores in the treatment group. By contrast, the placebo treatment group demonstrated no improvement across any of these measures.
Interestingly, both of the groups (treatment and placebo) experienced benefits with regards to improved sleep quality. It should be noted that the researchers did not see any significant changes in the UPDRS Part III which assesses motor performance (but this was a very short study – just 2 weeks).
In addition to this example, there have been other studies examining light therapy in Parkinson’s. For example, in 2017 this report was published in JAMA Neurology:
 Title: Timed Light Therapy for Sleep and Daytime Sleepiness Associated With Parkinson Disease: A Randomized Clinical Trial.
Title: Timed Light Therapy for Sleep and Daytime Sleepiness Associated With Parkinson Disease: A Randomized Clinical Trial.
Authors: Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC.
Journal: JAMA Neurol. 2017 Apr 1;74(4):411-418.
PMID: 28241159 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers were focused on determing if light theray could have any impact on excessive daytime sleepiness associated with Parkinson’s. They recruited 31 people with Parkinson’s (who were receiving stable dopaminergic therapy) with co-existing excessive daytime sleepiness (as defined by an Epworth Sleepiness Scale score of 12 or more). The participants were randomised (1:1) to a bright light therapy group (treatment; 16 people in this group; 10000 lux) or a dim-red light therapy (control; 15 people in this group; 300 lux) group. They were treated twice daily for 1-hour over 14 days.
The investigators found that bright light therapy resulted in significant improvements in excessive daytime sleepiness (as determined by change in Epworth Sleepiness Scale score – the average score at baseline was 15.81, which improved to 11.19 after the study. Interestingly, the researchers also found that light therapy was associated with increased daily physical activity. The researchers concluded that “light therapy may be a feasible intervention for improving sleep and alertness in patients with Parkinson’s“. And they added that “Further studies are required to determine optimal parameters of light therapy for Parkinson’s” – which I think is an important statement.
There is currently a lot of variablity between the available with regards to parameters, and more research is required on how to optimise
Still, impressive results though, right?
Yes, I agree. There may be a placebo response occurring in some of the control groups, but the fact that the treatment groups perform better is interesting.
But how?!? How could light be improving Parkinson’s?
Most of the researchers involved in these studies point towards the circadian system as being involved in the improvements that are being observed (particularly the improvements in sleep).
Circadian system?
The word ‘circadian’ comes from the Latin circa, which means “around”, and diēm, meaning “day”. Each of us has a circadian rhythm, which is an internal process that regulates the sleep-wake cycle and repeats roughly every 24 hours.
 Source: Wikipedia
Source: Wikipedia
This rhythm is controlled by certain biological processes in the brain that can be affected in Parkinson’s.
The eyes are the primary gate way to our response to light. Receptors in the eye (called melanopsin receptors) respond to light stimulation by sending a message to a region of the brain called the hypothamalus – specifically to a group of neurons referred to as the suprachiasmatic nucleus (or SCN). The SCN is considered the central pacemaker of the body, which gives us a sense of what time of the day it is.
 Source: Natap
Source: Natap
Activation of the SCN causes it to inhibit production of melatonin in the pineal gland. Melatonin is the hormone that tells our bodies that it is night time.
The skin is also receptive to light and can influence circadian rhythms.
The basic theory of light therapy is that it is helping to better synchronise circadian rhythms. A strong dose of light therapy helps the body to know what time of the day it is.
Ok, that might help with getting better sleep or excessive daytime sleepiness, but what about the improvements in movement in the PhotoPharmic study?
So this is where I get a bit lost for an explanation.
There is evidence that dopamine metabolism appears to be regulated by proteins involved in circadian rhythms (Click here to read more about this). Such circadian proteins include PER1 and PER2 – click here to read more about this). Perhaps by better synchronising circadian rhythms, light therapy is improving the activity of circadian proteins. And by improving the regulation of circadian proteins, maybe dopamine metabolism is being enhanced?
There appears to be a lot of different theories on this and some clarity (via a better understanding of the biology) would be helpful.
For those interested, click here and here to read more on this topic.
So what does it all mean?
Here at the SoPD, I get contacted by a lot of people and some of them have what I would politely refer to as “left field” ideas. But I am extremely open to them (as long as parties are not seeking to profit from them) for two reasons:
- for all the advances the world of research has made (in seeking a improved treatments and improved understanding about the condition), we are still missing big parts of the answer and we have to be open to dogma defying alternatives.
- if a light bucket helps somebody (placebo effect or otherwise), who am I to suggest otherwise.
The PhotoPharmic results have attracted some renewed attention to this field of light therapy. And while light therapy may not lead to a “curative” treatment, it certainly may help to improve quality of life for some members of the Parkinson’s community (particularly with regards to sleep and excessive daytime sleepiness. Thus, it is of interest here at the SoPD.
Given individuals differences in the circadian rhythms and the tremendous variation in symptoms between people with Parkinson’s, improving the calibration of light therapy parameters will be challenging and there may not be a perfect level for “Parkinson’s”. But if it can improve quality of life for sufferers, it is certainly worth exploring further.
We’ll keep an eye on this area.
…
…
…
…
…
…
…
Why are you still reading?
…
…
…
…
…
…
…
We’re finished.
…
…
…
…
…
…
…
Seriously, go away.
…
…
…
…
…
…
…
Alright.
So there might be a little bit more to discuss here.
This is Prof Li-Huei Tsai:
Li-Huei Tsai. Source: Bostonmagazine
She is the director of the Picower Institute for Learning and Memory in the Department of Brain and Cognitive Sciences at the Massachusetts Institute of Technology.
Prof Tsai and her colleagues have been doing some really interesting research in the field of neurodegeneration… and it involves flickering light.
In 2016, her research group published this report:
 Title: Gamma frequency entrainment attenuates amyloid load and modifies microglia.
Title: Gamma frequency entrainment attenuates amyloid load and modifies microglia.
Authors: Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai LH.
Journal: Nature. 2016 Dec 7;540(7632):230-235.
PMID: 27929004 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers noted that genetically engineered mice (5XFAD mice) which produce high levels of the Alzheimer’s associated beta amyloid, exhibit a reduction in a special kind of brain activity called gamma oscillations.
What are gamma oscillations?
In your brain, there are an enormous number of neurons communicating with each other on a seemingly continuous basis. This activity generates a lot of electrical activity which can be measured using special equipment called an electroencephalogram (ECG). ECG assesses electrical activity over a particular area of the brain.
The activity of neurons in the brain can become synchronised, in what we commonly refer to as “brain waves” (or oscillations). And these brain waves can be grouped based on their frequency. This frequency is measured in hertz (Hz) or cycles per second (or cps) and can reflect behaviour or level of cognitive activity:
Source: Hubpages
Activity in the gamma waves/oscillations is 30-120 Hz (or cps) and it is usually associated with high levels of brain activity, such as when we are learning.
Prof Tsai and her colleagues found that these gamma oscillation were reduced in the Alzheimer’s mice. Importantly, they noted that these changes occur before the mice start displaying the first pathological features of the disease.
What are the pathological features of Alzheimer’s?
Alzheimer’s is the most common neurodegenerative disease, accounting for 60% to 70% of all cases of dementia. It is a progressive neurodegenerative condition affecting approximately 30 million people around the world.
The condition is characterised by a global loss of cells in the brain.
 The brain on the right had Alzheimer’s. Source: BrainRepair
The brain on the right had Alzheimer’s. Source: BrainRepair
Inside the brain, in addition to the cell loss, there are two cardinal pathological features of the Alzheimer’s brain:
- Neurofibrillary tangles
- Amyloid plaques
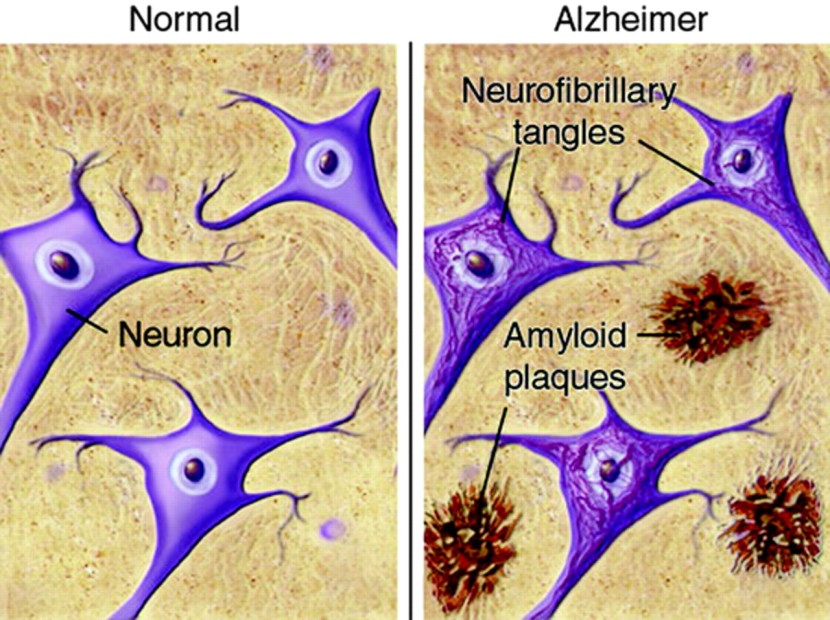
A schematic demonstrating the difference between healthy and Alzheimer’s affected brains. Source: MmcNeuro
The tangles are the clustering (or aggregation) of a protein called Tau (Click here to read a previous SoPD post on Tau). These tangles reside within neurons initially, but as the disease progresses the tangles can be found in the space between cells.
Amyloid plaques are clusters of protein that appear outside of the cells in Alzheimer’s. A key component of the plaque is a protein called beta amyloid.
Beta-amyloid has long been considered to be the villain in Alzheimer’s.
It is a piece of a larger protein that sits in the outer wall of nerve cells where it has certain functions. In certain circumstances, specific enzymes can cut it off and it floats away.
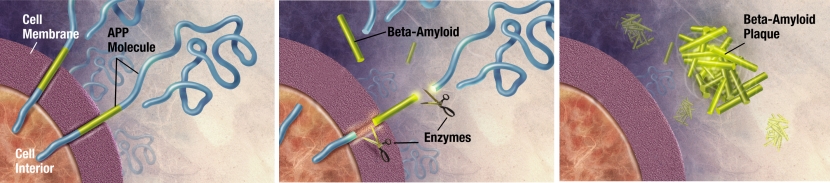
The releasing of Beta-Amyloid. Source: Wikimedia
Beta-amyloid is a very “sticky” protein and it has been believed that free floating beta-amyloid proteins begin sticking together, gradually building up into the large amyloid plaques. And these large plaques were considered to be involved in the neurodegenerative process of Alzheimer’s, causing stress and inflammation in the brain.
Thus, for a long time scientists have attempted to reduce the amount of free-floating beta-amyloid in the brain.
So the mice began to have less…gamma oscillations before they developed these amyloid plaques?
That is what the researchers found. And they noted that this reductuion in gamma synchronization in the context of Alzheimer’s has been previously reported by other research groups, both in animal models (Click here to read an example of this) and in humans with Alzheimer’s (Click here to read more about this).
But then the researchers began to ask “can we restore gamma oscillations?” and more importantly, “could this help with Alzheimer’s?“
And did they investigate these ideas?
They did.
Using optogenetics – which involves engineering neurons in the brain to become activated by a specific frequency of light (Click here to read a previous SoPD post explaining optogenics) – the researchers were able to increase gamma oscillations in the brain of mice.
And they found that this gamma stimulation reduced beta amyloid protein production in the 5XFAD Alzheimer’s mice.
 When the investigators looked at the brains of the mice they found less build up in mice exposed to 40Hz gamma stimulation than non-treated mice. In addition, they observed that the shape (or morphology) of the resident immune cells in the brain – called microglia – was different in the 40Hz stimulated mice.
When the investigators looked at the brains of the mice they found less build up in mice exposed to 40Hz gamma stimulation than non-treated mice. In addition, they observed that the shape (or morphology) of the resident immune cells in the brain – called microglia – was different in the 40Hz stimulated mice.
The gamma stimulation appeared to be activating the microglia in the brain, initiating them to swell up and clear the beta amyloid build up.
Wow! That’s amazing. But how can this be done in humans if the stimulation needs to be done inside the brain?
Yeah, it’s a bit of a problem. But the researchers were thinking the same thing.
They decided to try the obvious solution to this issue and simply expose mice to flickering lights (40 Hz oscillations for 1 hour) to see if it could restore gamma oscillations in their brains. You can see an example of a mouse being exposed to this ‘strobe-like’ stimulation by downloading “Extended Data Video 4.” from the study (Click here to find the file).
To their surprise, not only did this flickering light restore gamma oscillations in the brain, but it also reduced levels of beta amyloid in their brains. In fact, the researchers found that 7 days of 40 Hz flickering for 1 hour per day reduced levels of beta amyloid in the brains of the mice by 50%!
If none of this is making any sense, watch this video (kindly provided by MIT):
Since that initial study, the MIT researchers have been very busy further exploring this phenomenon, and earlier this year they published this report, which suggested that it wasn’t just light that could stimulate gamma oscillations in the brain, but also sound:
 Title: Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition.
Title: Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition.
Authors: Martorell AJ, Paulson AL, Suk HJ, Abdurrob F, Drummond GT, Guan W, Young JZ, Kim DN, Kritskiy O, Barker SJ, Mangena V, Prince SM, Brown EN, Chung K, Boyden ES, Singer AC, Tsai LH.
Journal: Cell. 2019 Apr 4;177(2):256-271.
PMID: 30879788
In this study, Prof Tsai and her collaborators designed an auditory tone stimulation approach that could drive gamma frequency neural activity in the brains of mice. And they found that 7 days of this auditory stimulation not only reduced beta amyloid protein levels in the brains of 5XFAD mice, but also improved memory function as well.
In addition, the investigators reported changes in vasculature – the blood vessels in the brain were wider following auditory stimulation.
Two important observations from this study:
- The interesting clearance effect was not restricted to beta amyloid protein, but it also had an impact on another protein associated with neurodegeneration called Tau (Click here to read a previous SoPD post on Tau). As far as I am aware no one has tested this approach on any Parkinson’s models (and I would be very pleased to be corrected on this!).
- The effect appears to be transcient. When the researchers examined the brains of mice just one week after the treatment had been stopped, they found that the activated microglia had returned to their normal shape.
This noninvasive technique is now being referred to as GENUS – short for “gamma entrainment using sensory stimulus” – and there is an interesting discussion about it over at the Alzforum website (Click here to read that post).
Not to stop there, Prof Tsai and her team have very recently published this report:
 Title: Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection.
Title: Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection.
Authors: Adaikkan C, Middleton SJ, Marco A, Pao PC, Mathys H, Kim DN, Gao F, Young JZ, Suk HJ, Boyden ES, McHugh TJ, Tsai LH.
Journal: Neuron. 2019 Jun 5;102(5):929-943.
PMID: 31076275
In this study, the researchers demonstrated that long-term daily GENUS treatment (from an early stage of neurodegeneration) may be neuroprotective, resulting in a preservation of neuronal health across various areas of the brain and improved cognitive performance in mice. Analysis of the neurons (transcriptomic and phosphoproteomic data) indicated that this chronic treatment improving neuronal function, enhancing neuroprotective features, and reduced levels of DNA damage in neurons. In addition, it also reduced the inflammatory properties of microglia in the brain.
It will be interesting to see what kind of turn this research will take next.
Has there been any effort to clinically test this GENUS effect in humans?
So Prof Tsai and her MIT colleagues have set up a biotech firm to further explore this potential therapeutic approach.
That company is called Cognito Therapeutics.
In April, 2018, Cognito Therapeutics began a Phase I trial called “Overture”, which was designed to evaluate the “GammaSense Stimulation device” (I have no idea what this looks like). 60 participants with early Alzheimer’s or mild cognitive impairment were recruited for this study and received 1 hour of GENUS, or sham treatment, daily for six months.
The study is a randomised, controlled, single-blind multi-center designed to assess safety of and adherence to the treatment. The primary endpoint (the measure by which the trial will be evaluated) is a Alzhiemer’s cognitive score (ADAS-Cog) and the secondary endpoint is brain imaging of beta amyloid to assess any reduction (Click here to read more about this study).
In November 2018, a second study called “Flicker” was started to evaluate the tolerability of a combined audio-visual GENUS stimulation. 10 people with early Alzheimer’s will be assessed for 1 hour of treatment per day. Five participants will receive eight weeks of GENUS, while the other five will receive four weeks of no treatment, followed by four weeks of GENUS (Click here to read more about this study).
In addition to those trials, earlier this year (May 2019), a second Phase 1 study called “Etude” was initiated comparing different dosing paradigms for auditory and visual GENUS (different number of times per day, different lengths of treatment, etc). This study is being conducted in 20 people with early Alzheimer’s or mild cognitive impairment and is being conducted over 1 year (Click here to read more about this study).
The results of these studies should be available in 2020.
Something to look out for in the new year?
Yes, but – and here comes the ‘managing expectations’ portion of the post – there has been an independent pilot clinical study, which did not find any beneficial effect from 40Hz light therapy in people with Alzheimer’s:
 Title: The Effect of 40-Hz Light Therapy on Amyloid Load in Patients with Prodromal and Clinical Alzheimer’s Disease.
Title: The Effect of 40-Hz Light Therapy on Amyloid Load in Patients with Prodromal and Clinical Alzheimer’s Disease.
Authors: Ismail R, Hansen AK, Parbo P, Brændgaard H, Gottrup H, Brooks DJ, Borghammer P.
Journal: Int J Alzheimers Dis. 2018 Jul 30;2018:6852303.
PMID: 30155285 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers were seeking to test the effect of a short-duration 40 Hz light flicker treatment regime in 6 people with Alzheimer’s. The participants received 10 days of treatment (2 hours per day exposure). They underwent a brain imaging session at the start and end of their 10 days of treatment to assess how much beta amyloid was in their brains (PiB PET imaging).
The researchers found no significant decrease in beta amyloid levels in any regions of the brain (see image below). They suggested that perhaps longer periods of treatment may be necessary to see clearance of beta amyloid from the human brain.
 Source: PMC
Source: PMC
We will wait to see the outcomes of the 3 Cognito Therapeutics trials before we start making any conclusions about GENUS.
I have to admit though that I am intrigued…
For those interested – click here for further reading on this topic.
So what does it all mean (2.0)?
For some time I have been asked by readers to write about a post about the photomodulation or light therapy field, but it is hard to know where to begin. There are many different types of devices being evaluated for Parkinson’s (and other forms of neurodegeneration), and a variety of different parameters are being tested. As a result, it has been difficult to put this post together (and hence the ridiculous length and convoluted nature of it!).
But I would reiterate that – as we wait for the outcomes of various clinical trials – if members of the Parkinson’s community are deriving benefits from sitting in front of a light therapy box, then more power to them. This statement should not be seen as a license for light therapy companies to pray on the PD community, but rather it should be taken as a call for the research world to be more open to left field ideas coming out of places like Tasmania.
Listen to the patients.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like!
The banner for today’s post was sourced from PhotoPharmic






Very interesting , Simon. Thank you for the research and the post. It seems like you could look for corroboration from various other epidemiological observations. For example, if the light level (lux) is the key, then it seems you should see a seasonal correlation with onset of Parkinson’s (or Alzheimer’s), or perhaps a higher risk probability in Alaska or Scandinavia than in Arizona or Greece. If the flicker frequency matters, then perhaps people who watch a lot of ‘telly’ with a flicker rate of 50-60 hertz would show a lower prevalence ( highly doubtful from my observations). If it’s the light wave frequency (or color) that matters , then office workers under fluorescent lights ( much bluer) should be less prone to Alzheimer’s than farm workers or construction workers who get lots of full-spectrum irradiation. I have noted I one of your previous posts that Nebraskans have roughly double the incidence rate (of Parkinson’s) compared with the general population. Does this hold for Alzheimer’s too?
LikeLike
Hi Tom,
Thanks for your interesting comment. I have to admit I have no idea about Alzheimer’s and light therapy. The epidemiological angle is interesting though: There is evidence that red heads have a higher frequency of Parkinson’s (https://scienceofparkinsons.com/2015/12/06/red-hair-sir-in-my-opinion-is-dangerous/), but many of the red heads live in more northern nations (Scotland, Scandinavia, etc) which have long days of sunlight during the summer and normal/average incidence of Parkinson’s. Perhaps those long days of sun light protect some of those individuals who might be at risk? I am of course wildly speculating here. I am not sure about the TV scenerio, but it is a curious observation.
Kind regards,
Simon
LikeLike
Several people with Par kinsons, including me, mention that symptoms are worse in the winter. So investigating light makes complete sense to me.
I am conducting my own experiment … I will be escaping the UK January gloom, visiting Hawaii and joining thousands of “snow birds” from northern states of US who travel to Florida every winter. they must know something! I’ll send a post card with the results.
Seriously though, it is why we are travelling in winter. As you say science can be stimulated by any number of ideas, even common sense.
LikeLike
100% agreed with the previous comment. The other half (with PD) seems to need to go out of the house for a long walk after breakfast. He has always been an exercise junky. Without this he has no motivation to do anything else. After a long walk he can settle and do other things. However it is not just the exercise – indoor exercise just does not work. Also gray days and short winter days certainly make symptoms worse. So light intensity and natural light seem to be very critical.
Have there been any studies of progression rate vs average time spent outdoors? Postal workers with PD vs academics?
On a separate note – the Spectramax story sounds a bit of a cheat – is that not just an LED screen on blank? – the frequency sounds familiar… think of all of the fuss about excessive screen time in the evening, especially for children.
The light and / or sound pulsation story seems to have been taken up by phone App producers and YouTube. I tried some myself and found that most made my scalp crawl and induced a headache. However some of the offerings from the meditation world which involved more than just the 40Hz pure tone were OK to fall asleep to, or have on in the background.
If the 40hz story is real, then I guess infra red / warmth pulses make sense. Certainly more pleasant than the pure 40Hz sound. Alternatively light absorption into blood veins may do something – in which case i wonder if it works better on people with less hair? Maybe a light muffler rather than a hat would work better?
Anyway a great story!
And fantastic that people are checking stuff for themselves. Far too much lowish tech research is not being done because there is no profit in it.
LikeLike
Hi Eirwen,
Thanks for your comment – very envious of your winter migration plans. It is true that symptoms fluctuate with the seasons – even non-motor (a recent example of research on this – https://jnnp.bmj.com/content/90/3/e24.2). Perhaps we need a little more common sense to our approach to Parkinson’s research 🙂
Kind regards,
Simon
LikeLike
Simon, You seem to have ignored the science behind the Red light buckets/coronet. Do we assume you looked at the science and dismissed it as placebo/rationalisation? I use a coronet so am interested.
Thanks, Dave Thomson
LikeLike
Hi Dave,
Thanks for your comment. I am aware of the red light work, but please don’t assume that I dismissed it. This post was rather long so I simply had to cut and choose what to keep in it. I will come back to red light in a future post.
Kind regards,
Simon
LikeLike