
|
Researchers from Düsseldorf (Germany) and a biotech company in San Francisco have published a research report in which they present evidence that the H1N1 influenza A virus can cause the Parkinson’s-associated protein alpha synuclein to start aggregating in neurons (both in cell culture and mice). In addition, they reported that an influenza drug blocked this aggregation effect. In today’s post, we will discuss what H1N1 influenza virus is, what the new research report found, and we will consider what this means for Parkinson’s.
|
 Source: Ucf
Source: Ucf
The date today is the 11th March, 2020.
Based on the news headlines trending on all forms of media, it is probably the absolute worse moment to consider writing a post about how a particular viral infection could be playing a role in Parkinson’s,… but here we are.
Over night a research report was published that immediately grabbed my attention.
This is the report:
 Title: Disruption of cellular proteostasis by H1N1 influenza A virus causes α-synuclein aggregation.
Title: Disruption of cellular proteostasis by H1N1 influenza A virus causes α-synuclein aggregation.
Authors: Marreiros R, Müller-Schiffmann A, Trossbach SV, Prikulis I, Hänsch S, Weidtkamp-Peters S, Moreira AR, Sahu S, Soloviev I, Selvarajah S, Lingappa VR, Korth C.
Journal: Proc Natl Acad Sci U S A. 2020 Mar 9. pii: 201906466. [Epub ahead of print]
PMID: 32152117
In this study, the researchers reported that infection by the H1N1 influenza A virus (very different to the current coronavirus COVID-19) can cause accumulation and aggregation of the Parkinson’s-associated protein alpha synuclein.
Remind me, what is H1N1 influenza?
Influenza is a single-stranded, RNA virus of the orthomyxovirus family of viruses.

A schematic of the influenza virus. Source: CDC
It is the virus that causes ‘the flu’ – (runny nose, sore throat, coughing, and fatigue) – with the symptom arising two days after exposure and lasting for about a week. In humans, there are three types of influenza viruses, called Type A, Type B, and Type C. Type A are the most virulent in humans.
The influenza virus behind both of the outbreaks in the 1918 pandemic was a Type A.
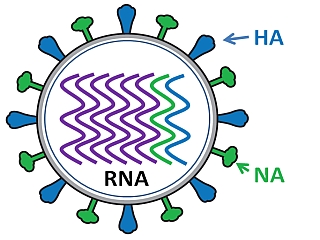
Schematic of Influenza virus. Source: Bcm
As the image above indicates, the influenza virus has a rounded shape, with “HA” (hemagglutinin) and “NA” (neuraminidases) proteins on the outer surface of the virus. The HA protein allows the virus to stick to the outer membrane of a cell. The virus can then infect the host cell and start the process of reproduction – making more copies of itself. The NA protein is required for the virus to exit the host cell and go on to infect other cells. Different influenza viruses have different combinations of hemagglutinin and neuraminidase proteins, hence the numbering. For example, the Type A virus that caused the outbreaks in the 1918 pandemic was called H1N1.
Inside the influenza virus, there are there are eight pieces (segments) of RNA, hence the fact that influenza is an RNA virus. Some viruses have DNA, while others have RNA. The 8 segments of RNA provide the information that is required for making new copies of the virus. Each of these segments provides the instructions for making one or more proteins required for making the virus (eg. segment 4 contains the instructions to make the HA protein).
 The 8 segments of RNA in influenza. Source: URMC
The 8 segments of RNA in influenza. Source: URMC
The Influenza virus is one of the most changeable viruses we are aware of, which makes it such a tricky beast to deal with. Influenza uses two techniques to change itself over time.
These techniques are called shift and drift.
Shifting is an sudden change in the virus, which produces a completely new combination of the HA and NA proteins. Virus shift can take place when a person or animal is infected with two different subtypes of influenza. When new viral particles are generated inside the cell, there is a mix of both subtypes of virus which gives rise to an all new type of virus.

An example of viral shift. Source: Bcm
Drifting is the process of random genetic mutation. Gradual, continuous, spontaneous changes that occur when the virus makes small “mistakes” during the replication of its RNA. These mistakes can results in a slight difference in the HA or NA proteins, and although those changes are small, they can be significant enough that the human immune system will no longer recognise and attack the virus. This is why you can repeatedly get the flu and why new flu vaccines must be administered each year to combat the novel forms of circulating influenza virus.
|
RECAP #1: Researchers in Germany report that a strain of the influenza virus causes clustering of the Parkinson’s-associated protein, alpha synclein. Influenza is an RNA virus that is responsible for the common ‘flu’.
|
Interesting. So what exactly did the researchers find?
Firstly, the scientists treated human dopamine neurons being grown in cell culture with a low dose of H1N1 influenza A virus (so that 30-40% of the cells were infected). 24 hours later, they observed no morphological changes in the cells, but the number of alpha synuclein aggregates were significantly higher in the treated cells (than non-treated/infected cells).
What is meant by alpha synuclein aggregates?
Alpha synuclein gets a lot of discussion on this website. It is a protein in the brain that is intimately associated with Parkinson’s.
As a newly minted protein, it usually looks like this:
 Alpha synuclein. Source: Wikipedia
Alpha synuclein. Source: Wikipedia
By itself, alpha synuclein is considered a monomer, or a single molecule that will bind to other molecules to form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s disease, alpha-synuclein also aggregates to form what are called ‘fibrils’.

Microscopic images of Alpha Synuclein (AS) monomers, oligomers and fibrils. Source: Brain
Oligomer versions of alpha-synuclein are emerging as having a key role in Parkinson’s. They can lead to the generation of fibrils and may cause damage by themselves.

Source: Nature
As a result of this potential toxic effect, numerous therapeutic approaches are being clinically tested that have anti-alpha synuclein properties (some of which are reviewed in this SoPD post).
|
RECAP #2: Alpha synuclein is a very common protein in the brain, making up about 1% of the protein in neurons. In Parkinson’s, alpha synuclein protein clusters (or aggregates) together in the brain, and this behaviour may be involved in the neurodegeneration associated with the condition.
|
Ok, so the influenza virus causes alpha synuclein to cluster together in cells in culture?
Yes, the researchers demonstrated this in multiple types of neurons – not just dopamine neurons. And alpha synuclein was not the only protein affected. The investigators found that a protein called “Disrupted-in-schizophrenia 1” (or DISC1) was also aggregating 24 hours after H1N1 influenza A virus infection.
Interesting to note that alpha synuclein and DISC1 did not co-aggregate together.
Also interesting to note that additional aggregation proned proteins that are associated with other neurodegenerative conditions did not aggregate during H1N1 influenza A virus infection. Specifically, the investigators reported that Alzheimer’s-associated Tau or frontotemporal dementia-/amyotrophic lateral sclerosis (ALS)-associated TDP-43 did not aggregate in infected cells, suggesting the possibility of a protein-selective effect of the viral infection.
Next the investigators shifted away from cell culture and looked in the brains of H1N1 influenza A virus infected mice.
 The researchers infected the mice (via the nose), and 28 days later they found that levels of both alpha synuclein and Disc1 (but not of Tau and TDP-43) had increased significantly in brains of infected mice.
The researchers infected the mice (via the nose), and 28 days later they found that levels of both alpha synuclein and Disc1 (but not of Tau and TDP-43) had increased significantly in brains of infected mice.
Of particular interest in this study is that the researchers reported that Oseltamivir administration significantly decreased in the percentage of alpha synuclein aggregates in infected cells.
What is Oseltamivir?
Oseltamivir, sold under the brand name Tamiflu, is an antiviral medication used to treat and prevent influenza A and influenza B.
Source: ClickPharm
Where can I get me some Tamiflu?
Before you get too excited, there have been reports of Tamiflu causing dyskinesias in some cases of Parkinson’s (Click here for an example), and overall there has been very little research exploring this medication in the context of Parkinson’s.
But there is already an anti-influenza medication that is already clinically approved for Parkinson’s.
Which is?
Amantadine.
 Source: NDC
Source: NDC
Sold under the brand name Gocovri, it is effective against all influenza A subtypes (H1N1, H2N2 and H3N2). Amantadine blocks the influenzavirus A M2 proton channel, which prevents newly produced viruses from being released.
And it is used in Parkinson’s as a therapy for dyskinesias and rigidity.
|
RECAP #3: The H1N1 influenza A virus infection caused alpha synuclein protein to aggregate, but not other proteins associated with neurodegenerative conditions, like Alzheimer’s and ALS. The researchers reported that treating infected cells with Tamiflu – an antiviral medication – reduced the increase in alpha synuclein levels.
|
Interesting. But has anyone else ever reported influenza having an effect in the context of Parkinson’s?
Yes.
This is Dr Richard J Smeyne:

Source: Researchgate
He is a Professor of neuroscience at Thomas Jefferson University in Philadelphia.
And his research group has published several interesting research reports on this topic, including:

Title: Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration.
Author: Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R, Smeyne RJ.
Journal: Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):14063-8.
PMID: 19667183 (This article is OPEN ACCESS if you would like to read it)
Dr Smeyne and his colleagues found in this study that when they injected the highly infectious A/Vietnam/1203/04 (H5N1) influenza virus into mice, the virus progressed from the periphery (outside the brain) into the brain itself, where it induced Parkinson’s-like symptoms.
The virus also caused a significant increase in the accumulation of alpha synuclein. In addition, they witnessed the loss of dopamine neurons in the midbrain of the mice at 60 days after the infection – that cell loss resembling what is observed in the brains of people with Parkinson’s.
Naturally this got the researchers rather excited!
In a follow up study on H5N1, however, these same researchers found that the Parkinson’s-like symptoms that they observed were actually only temporary:

Title: Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice.
Authors: Jang H, Boltz D, McClaren J, Pani AK, Smeyne M, Korff A, Webster R, Smeyne RJ.
Journal: Journal for Neuroscience, 2012 Feb 1;32(5):1545-59.
PMID: 22302798 (This article is OPEN ACCESS if you would like to read it)
Dr Smeyne and colleagues repeated the 2009 study and had a closer look at what was happening to the dopamine neurons that were disappearing at 60 days post infection with the virus. When they looked at mice at 90 days post infection, they found that the number of dopamine neurons had returned to their normal number. This pattern was also observed in a region of the brain called the striatum, where the dopamine neurons release their dopamine. The levels of dopamine dropped soon after infection, but rose back to normal by 90 days post infection.
How does that work?
The results suggest that rather than developing new dopamine neurons in some kind of miraculous regenerative process, the dopamine neurons that were infected by the virus simply stopped producing dopamine while they dealt with the viral infection. Once the crisis was over, the dopamine neurons went back to life as normal. And because the researcher use chemicals in the production of dopamine to identify the dopamine neurons, they mistakenly thought that the cells had died when they couldn’t see those chemicals. We have recently explored this idea of dopamine neurons not actually dying in a SoPD post exploring other research (Click here to read that post).
One interesting observation from the study was that H5N1 infection in mice induced a long-lasting inflammatory response in brain. The resident helper cells, called microglia, became activated by the infection, but remained active long after the dopamine neurons returned to normal service. The investigators speculated as to whether this activation may be a contributing factor in the development of neurodegenerative disorders.
In a follow up study, they investigated this further by looking at another influenza virus that doesn’t actually infect cells in the brain:

Title: Induction of microglia activation after infection with the non-neurotropic A/CA/04/2009 H1N1 influenza virus.
Author: Sadasivan S, Zanin M, O’Brien K, Schultz-Cherry S, Smeyne RJ.
Journal: PLoS One. 2015 Apr 10;10(4):e0124047.
PMID: 25861024 (This article is OPEN ACCESS if you would like to read it)
In this study, a different type of influenza (H1N1) was tested, and while it did not infect the brain, it did cause the microglia cells to flare up and become activated. And again, this activation was sustained for a long period after the infection (at least 90 days).
|
NOTE: The H1N1 virus used in this study (A/California/04/2009 H1N1) is different to the H1N1 virus used in the study that today’s post is based on (which is the A/WSN/33 H1N1). The latter does infect cells in the brain (Click here to read more about this).
|
This is a really interesting finding and relates to the idea of a “double hit” theory of Parkinson’s, in which the virus doesn’t necessarily cause Parkinson’s but may play a supplemental or distractionary role, grabbing the attention of the immune system while some other toxic agent is also attacking the body. Or perhaps simply weakening the immune system by forcing it to fight on multiple fronts. Alone the two would not cause as much damage, but in combination they could deal a terrible blow.
But why would alpha synuclein be aggregating in the presence of a virus?
Well, alpha synuclein has been shown to have anti-viral properties:

Title: Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain.
Authors: Beatman EL, Massey A, Shives KD, Burrack KS, Chamanian M, Morrison TE, Beckham JD.
Journal: J Virol. 2015 Dec 30;90(6):2767-82. doi: 10.1128/JVI.02949-15.
PMID: 26719256 (This article is OPEN ACCESS if you would like to read it)
David Beckham (not the football player) and his research colleagues introduced West nile virus to brain cells grown in cell culture and they observed an increase in alpha synuclein production. They also found that the brains of people with West nile infections had increased levels of alpha synuclein.
The researchers then injected West Nile virus into both normal mice and genetically engineered mice (which produced no alpha synuclein) and they found that the genetically engineered mice which produced no alpha synuclein died quicker than the normal mice. They reported that there was an almost 10x increase in viral production in the genetically engineered mice. This suggested to them that alpha synuclein may be having a protective effect in infected cells.
Interesting. So alpha synuclein could be protecting the cell against a viral infection?
Yes, but the new research report from the scientists in German that we reviewed at the top of this post found something else that could explain the aggregation of alpha synuclein.
What did they find?
They reported that H1N1 influenza A virus infection caused a disruption in the waste disposal system of the cell (called autophagy), and they proposed that this was the cause of the build up and aggregation of alpha synuclein.
Ok, so do you think flu vaccination could be a means of blocking or slowing Parkinson’s?
Dr Smeyne’s research group have explored this idea in this report:

Title: Synergistic effects of influenza and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can be eliminated by the use of influenza therapeutics: experimental evidence for the multi-hit hypothesis
Authors: Sadasivan S, Sharp B, Schultz-Cherry S, & Smeyne RJ
Journal: npj Parkinson’s Disease 2017 May 23;3:18.
PMID: 28649618 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers used a classical Parkinson’s mouse model, involving a neurotoxin called MPTP (a toxin that specifically kills dopamine neurons), and they found that infecting mice with the H1N1 virus resulted in a 20% greater loss of dopamine neurons than mice that were treated with MPTP alone.
But they also reported that this increase in dopamine neuron loss was completely eliminated by giving the mice the influenza vaccination. The researchers concluded that the results demonstrate that multiple insults (such as a viral infection and a toxin) can enhance the impact, and may even be significant in allowing an individual to cross a particular threshold for developing a disease.
But there is one problem with the flu vaccination idea: the vaccines change every year.
What? Why?
Most flu vaccines target three strains of the viruses (and are thus called ‘Trivalent flu vaccines’) which are selected each year based on data collected by various health organisations around the world.
The three chosen viruses for a particular year are traditionally injected into and grown in hens’ eggs, then harvested and purified before the viral particles are chemically deactivated. The three dead viruses are then pooled together and packaged as a vaccine. As you can see in the image below, the process of vaccine production is laborious and takes a full year:
The process of vaccine production. Source: Linkedin
By injecting people with the dead viruses from three different strains of the influenza virus, however, the immune system has the chance to build up a defence against those viruses without the risk of the individual becoming infected (the dead viruses in the vaccine can not infect cells).
Flu vaccines cause the immune system to produce antibodies which are used by the immune system to help defend the body against future attacks from viruses. These antibodies generally take about two weeks to develop in the body after vaccination.
As we have said most injected flu vaccines protect against three types of flu virus. Generally each of the three viruses is taken from the following strains:
- Influenza A (H1N1)
- Influenza A (H3N2)
- Influenza B
|
RECAP #4: A number of scientific reports have been published by an independent research team suggesting similar results to this new study and demonstrating that seasonal flu vaccination may also have beneficial effects. The problem, however, is that the seasonal vaccine changes from year to year (as do the strains of influenza).
|
Are there any biotech companies exploring this area?
Interesting question.
The research report that we have reviewed today was a collaborative effort between researchers in Düsseldorf (Germany) and a San Francisco-based biotech company called Prosetta Biosciences.
Prosetta has a stated interest in Parkinson’s on their website. They do not give away too much information on their website, so it will be interesting to see how this research develops and which way the company choses to go.
So what does it all mean?
I was in two minds about writing this post. It felt both timely and completely inappropriate. With everyone so worried about the current state of the world, I was nervous about sharing this new research report. But the science won the day and I decided that it was of genuine interest, so I pressed on. In addition, I am a wee bit biased as I have long been a fan of a pathogen-based theory of Parkinson’s and other neurodegenerative conditions.
Researchers in Germany have discovered that the infection of cells by a particular type of virus (influenza H1N1) causes an increase in the Parkinson’s associated protein alpha synuclein. The research presented in today’s post requires further exploration before we can make any definitive conclusions from it. And it probably would not explain every case of Parkinson’s.
In addition, it also begs the question: if influenza causes alpha synuclein accumulation and everyone gets infected by influenza from time to time, why do so few people actually develop Parkinson’s? This may point towards the biological processes that deal with cleaning up the mess after a viral infection not being optimal, rather than the infection itself. In other words, the infection may not be influential in Parkinson’s, but rather it might be issues with the clean up team that cause the trouble.
I hope readers found this as interesting as I did.
A comment on the current coronavirus (COVID–19) situation
I am by no means an expert on virology (so I will be careful what I write here), but I do appreciate that everyone is concerned about the current situation surrounding the COVID-19 pandemic.
I have only two things to say about the situation:
- The new virus is certainly nasty, but there is no reason to panic.
- It is important to take precautions and to seek information from appropriate sources.
The World Health Organisation has a great deal of information about the COVID-19 virus on their website, which is being regularly updated (Click here to see the WHO website). In addition, they have a lot of videos with useful tips on how to keep yourself and loved ones safe – such as this video:
For information relating to Parkinson’s and COVID-19, both Parkinson’s UK and the Michael J Fox Foundation have very good guidance pages dealing with the outbreak.
Be safe, and – as the words on the cover of “The Hitchhiker’s Guide to the Galaxy” (42nd anniversary this year) said – “don’t panic”.
 Source: Medium
Source: Medium

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE – The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by a research scientist, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. Please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Neuro-hemin



This may not be timely but it’s certainly informative. As a person diagnosed with early onset PD and a history of flu that resulted in a long recovery and subsequent health issues- I’ve always believed there is some pathogenic connection and/or as you suggest and inefficient “clean-up” process.
LikeLike
Hi Heather,
Thanks for your comment and thanks for sharing. Interesting to get anecdotal bits of potential insight. Glad you liked the post.
Kind regards,
Simon
LikeLike
I personally want to thank you for writing the article. While the coronavirus is very real and very dangerous, it will eventually pass. That can not be said about my wife’s Parkinsons. I love the information that you present. It keeps us up to date on the newest information, and provides hope. I have shared your site with a number of people all of which look forward to the things you post.
LikeLike
Could you clarify? Would amantadine then have the same effect as tamiflu?
LikeLike
Hi Di,
Thanks for your comment, but I’m afraid I can offer no clarity on this. Unfortunately neither have been tested in humans in the context of PD, so any answer would be guesstimation at best. Sorry.
Kind regards,
Simon
LikeLike
Thanks for this post. I experienced the worst flu in my life with a fever lasting almost a week in 2010. I recovered but felt a lingering unease about my health. First symptoms of what was diagnosed as PD in 2014 appeared late in 2013. I have often felt that this flu had triggered something connected to my diagnosis.
I always enjoy your posts even if I have to skim over some of the fabulous details.
LikeLike
Hi Cathy,
Thanks for your comment and for sharing. Glad you liked the post, not at all offended that you skim “some of the fabulous details” 🙂
Simon
LikeLike
Very interesting and timely summary! Thank you, Simon. For what it’s worth, I was one of the first in the UK to contract Swine Flu (a subtype of the H1N1 virus) in 2009, and I was diagnosed with Parkinson’s in 2013. However, like most people with PD, I started to develop symptoms 2-3 years before diagnosis. I wonder ….. !
LikeLiked by 1 person
Hi Ken,
Thanks for sharing your interesting comment. Very intriguing indeed.
Glad you liked the post.
Kind regards,
Simon
LikeLike
My husband who had no pre Parkinsons symptoms, was ill for a week with a fever and began shaking one week later.
LikeLike
An interesting thought… At least 3 of the substances mentioned as having possible use for Parkinson’s are also mentioned online as having potential in ameliorating Covid virus infections, and possibly as prophylaxis. Chloroquine (and quinine), licorice and baicalein. At least an excuse for another G&T while hiding at home as tonic contains quinine.
LikeLike
Another anecdote for the party… I had the flu pretty bad about 2-3 years pre diagnosis. Last time I had it was 15 years before and have similarly questioned if it was somehow related. Any data on year in/out flu shot recipients have a lower incidence?
LikeLike