|
# # # # Tiny variations in a region of DNA referred to as “Parkin” are associated with an increased risk of developing Parkinson’s (particularly young onset forms of the conditions). The Parkin DNA provide the instructions for making a protein that is involved with many functions inside cells. New research indicates that as we age, Parkin protein becomes less available. In fact, by the time we turn 50 years of age, “Parkin is largely insoluble”, meaning that the majority of the protein is no longer able to do its job. This shift appears to involve oxidation changes. In today’s post, we will discuss what Parkin and oxidation are, how Parkin might be affected by oxidation, and how this information might be useful to treating Parkin-associated Parkinson’s. # # # # |
 Me (I wish) before 27. Source: Pinterest
Me (I wish) before 27. Source: Pinterest
I don’t know about you, but 27 was my peak.
Before my 27th birthday, I could run around all over the place – acting like an idiot, with all the energy in the world. I was invincible and having lots of fun. And yes, some vices might have been involved – I would drink myself blind on a Friday night, wake up fresh the next day and do it all merrily again.
 Me before 27. Source: Thefix
Me before 27. Source: Thefix
But then, my 27th birthday came along and I woke up the next day tired and feeling… fatigued. Weary even. And definitely with less enthusiasm than I had before I passed out the night before. My father called it a “hang-over” (which up until that time I had naively/idiotically thought I was immune to).
 Me, before (left) and after 27 (right). Source: Wanna-joke
Me, before (left) and after 27 (right). Source: Wanna-joke
But I gradually developed this sinking feeling that it was something else.
Something more sinister.
It was as though something had changed. Something inside of me.
And I distinctly remember a moment of realisation, when I asked “Am I getting old???”
My father saw my concern and gave me sage advice (“It’s like I always say, aging ain’t for sissies“), and with that I changed my ways.
 Source: DS
Source: DS
Since that moment, I have been fascinated by the biology of aging, particularly in the context of Parkinson’s (age is the main correlate with neurodegenerative conditions like Parkinson’s and Alzheimer’s). So it was with great interest that I read a manuscript in November last year that had been posted on the openly-available preprint database bioRxiv.
What did the manuscript say?
Before we go any further, I have to say that I am about to be break one of the unwritten rules of science communication (…again).
Until a research report has been through the peer-review process you probably should not be discussing the results in the public domain. But in this particular case, the research is really interesting and relevant to what we discuss on this website. In addition, it has been made available on the OPEN ACCESS preprint depository website called BioRxiv, for the research community to share and discuss.
 Source: BioRxiv
Source: BioRxiv
I should also add that this is not the first time we have discussed manuscripts on BioRxiv (Click here, here, here and here to read other SoPD posts on Biorxiv manuscripts).
We are regular rule breakers here at the SoPD, and with that said, let’s move on.
Here is the manuscript in question:
 Title: Age-Associated Insolubility of Parkin in Human Midbrain is Linked to Redox Balance and Sequestration of Reactive Dopamine Metabolites
Title: Age-Associated Insolubility of Parkin in Human Midbrain is Linked to Redox Balance and Sequestration of Reactive Dopamine Metabolites
Authors: Tokarew JM, Kodsi DNE, Lengacher NA, Fehr TK, Nguyen AP, Shutinoski B, O’Nuallain B, Jin M, Khan J, Ng ACH, Li J, Jiang Q, Zhang M, Wang L, Sengupta R, Barber K, Tran A, Zandee S, Dong X, Scherzer CR, Prat A, Tsai E, Takanashi M, Hattori N, Chan JA, Zecca L, West A, Holmgren A, Puente L, Shaw GS, Toth G, Woulfe J, Taylor P, Tomlinson JJ, Schlossmacher MG
Database: bioRxiv
DOI: https://doi.org/10.1101/2020.11.20.392175
In this study, the researchers were interested in a Parkinson’s-associated protein called Parkin.
What is Parkin?
Sometimes referred to as PARK2 (as it was the second gene to be associated with Parkinson’s), Parkin is a region of DNA (a gene) that provides the instructions for making a protein that functions as a ubiquitin ligase.
The structure of Parkin protein. Source: Wikipedia
What is a ubiquitin ligase?
A ligase is an enzyme that initiates the joining of two molecules. It forms a new chemical bond between them.
Ubiquitin is a marvellous little protein that – as the name suggests – is ‘ubiquitous’ in all cells, and it affects all aspects of cell biology. It works its magic by being bound to proteins through a process known as ubiquitination.
 The structure of ubiquitin protein. Source: Wikipedia
The structure of ubiquitin protein. Source: Wikipedia
Ubiquitination can change the way a protein functions – like turning on a light switch – or it can be used to label an old/damaged protein for disposal.
This short video nicely explains ubiquitin:
It is important to understand that ubiquitin is involved in all kinds of biological processes, including:
- Apoptosis (cell death)
- Cell division and multiplication
- Degeneration of neurons and muscular cells
- DNA transcription and repair
- Immune and inflammatory response
- Stress response pathway
So Parkin attaches ubiquitin to other proteins and is involved with lots of cellular processes?
Exactly. Specifically, Parkin is an E3 ubiquitin ligase – which is the third step in ubiquitination that is described in the video above.
But how is Parkin associated with Parkinson’s?
As I briefly mentioned above, Parkin/PARK2 was the second gene to be associated with Parkinson’s (alpha synuclein/SNCA being the first). Tiny variations in the region of DNA (PARK2) that provide the instructions for making the Parkin protein are some of the most common genetic risk factors for individuals with young-onset Parkinson’s (or YOPD). These cases are typically diagnosed at some point under 40 years of age.
There isn’t one single Parkin genetic variation associated with Parkinson’s. Rather, there are currently 10 common mutations in the Parkin gene that can give rise to early-onset Parkinson’s. And it should be noted that Parkin has also been associated with different types of cancer – there are 13 cancer-related genetic variants (Parkin is recognised as a tumor suppressor – click here to read more about this).
Importantly, only two of the Parkinson’s related variants are associated with an increased risk of cancer (they are R24P and R275W – red+black arrow heads in the image below).

Comparing PARK2 Cancer and PD associated mutations. Source: Nature
Thus it is important to know exactly where your mutation is, if in fact you have one. And even if you have the R24P and R275W mutation, it does not necessarily mean that you are definitely going to develop a particular type of cancer. It simply means that you are slightly more predisposed to it than someone without that Parkin genetic variation (and please remember that a lot of genetic variations are required for a cancer to start!).
It is not something to worry about, but it is good to be aware of this association and to have regular check ups.
What does Parkin-associated Parkinson’s look like?
Individuals diagnosed with Parkin-associated YOPD are usually diagnosed around 30 years of age (but this can vary greatly). The condition is slower in progression than other forms of Parkinson’s and often presents itself clinically via tremor, bradykinesia, and lower-limb dystonia (sustained muscle contractions).
Affected individuals will generally have a preserved sense of smell and cognitive deficits are rare. Interestingly, at the postmortem stage, there are few reported cases with lewy bodies (one of the fundamental hallmarks of the Parkinsonian brain).
For those interested in learning more about PARKIN-associated YOPD, click here for a thorough review of the condition.
|
# RECAP #1: Tiny variations in the DNA that provides the instructions for making a protein called Parkin are some of the most common genetic risk factors for young onset Parkinson’s (diagnosed before 40 years of age). Parkin has many functions inside of cells, but it has primarily been associated with attaching another protein – called ubiquitin – to other proteins. # |
So the researchers were interested in Parkin. What were they specifically investigating?
Given the association with young-onset Parkinson’s, the researchers wanted to look at what was happening to Parkin protein over time in the body. They were basically asking: “how does the protein and its function change as we age?”
To answer this, they collected samples of postmortem samples of brain – primarily focusing on two regions of the brain: the midbrain and the cerebral cortex. The midbrain is the region where the dopamine neurons reside – these are the neurons badly affected by Parkinson’s – while the cerebral cortex covers a large area of the overlying brain – it’s the walnut-looking structure on top.
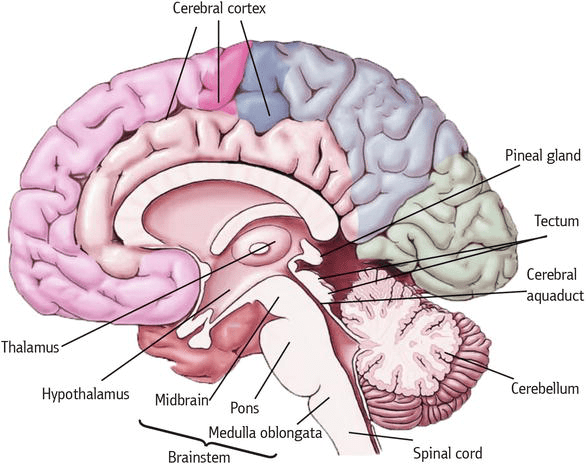 Areas of the brain. Source: Intechopen
Areas of the brain. Source: Intechopen
The samples from the midbrain came from 20 individuals of varying ages (the range was 26 – 82yrs), while more than 40 samples of cerebral cortex (age range 5 – 85 years) were also used in the study.
The investigators started by collecting Parkin protein from the samples… and this is where they discovered something really interesting:
In the samples from brains under the age of 20 years of age, approximately 50% of the Parkin protein was soluble in the experimental solutions used. But curiously this percentage dropped to less than 10% in the samples collected from brain over 50 years of age.
This means that most of the Parkin protein was insoluble in older brain samples.
What does “soluble” and “insoluble” mean?
A protein is soluble if it can dissolve in a solution forming a homogeneous solution. The correct functional properties of proteins depends on their solubility.
When Parkin becomes insoluble, it is no longer able to do its function.
So when you say “most of the Parkin protein was insoluble in older brain samples” that means that most of the Parkin protein isn’t functioning?
Yes, that is what the new research reports.
And the effect appears to be specific to the higher regions of the brain. Bizarrely, the researchers found that the levels of soluble Parkin protein remained stable across aging in samples of spinal cord. In addition – and even more strange – when the researchers looked at other proteins (including Parkinson’s-associated proteins like DJ-1 and alpha synuclein), they did not see this age-dependent solubility phenomenon.
Wow. That is really interesting.
Oh but wait, there’s more:
This age-dependent loss of solubility for Parkin protein also appears to be unique to the human brain – the researchers did not see the same effect in samples of aged brain from other species (including mice, rats, and monkeys).
Whoa!
Whoa, indeed.
And the effect is apparently occurring to the protein specifically, because in the brain samples there were no differences in the levels of RNA across the samples and ages examined. Cells appear to have no problem generating Parkin RNA, but something is happening to the protein itself.
In order to better understand this, the researchers next wanted to explore whether there were any possible associations between this curious age-dependent effect on Parkin protein and other factors associated with aging, such as oxidative changes.
What are oxidative changes?
Oxidation is the loss of electrons from a molecule, which in turn destabilises that particular molecule.
Think of iron rusting. Rust is the oxidation of iron – in the presence of oxygen and water, iron molecules will lose electrons over time. Given enough time, this results in the complete break down of objects made of iron.

Rusting iron. Source: Thoughtco
The exact same process occurs in biology. Molecules in your body go through a similar process of oxidation – losing electrons and becoming unstable. This chemical reaction leads to the production of what we call free radicals, which can then go on to damage proteins and cells.
As we age, there is an increase in oxidative change in our bodies – sorry to say this, but it is a battle that we are all loosing as we grow older (like my Dad says “Aging ain’t for sissies“).
In their analysis, the researcher found that certain agents that can cause oxidative stress (such as hydrogen peroxide) increase in concentration in the brain samples with age and were negatively correlated with Parkin solubility. What’s more, the scientists found that these oxidative conditions actually altered Parkin protein structure, leaving the protein highly oxidised and insoluble.
So oxidative conditions are reducing the amount of functional Parkin as we age?
That is what this new data is indicating.
The investigators next explored the properties of normal Parkin protein and found that it functions as an antioxidant.
What is an antioxidant?
While free radicals are the bad guys in oxidation, antioxidants can be considered the good guys. They are molecules that neutralize the free radicals by donating one of their own electrons. The antioxidant do not become free radicals by donating an electron because by their very nature they are stable with or without that extra electron.

How free radicals and antioxidants work. Source: h2miraclewater
In the Parkin study, the researchers found that normal Parkin protein functions as an antioxidant, and that it is able to reduce levels of hydrogen peroxide.
Interestingly, they also reported that Parkin boosts neuromelanin formation.
What is neuromelanin?
Neuromelanin is the brain-version of a pigment called melanin, which is found in the skin, eyes, and hair. It is the substance that gives skin & eyes their colour. Dark-skinned people have more melanin in their skin than light-skinned people.
In the brain, certain types of cells, such as the dopamine neurons, produce large amounts of neuromelanin.

Neuromelanin (the brown patches) in dopamine neurons. Source: Schatz
Neuromelanin appears in large quantities in the human brain, in much lesser amounts in some of the non-human primates, and is almost absent from the brain in many lower species (like mice and rats).
Dopamine neurons in the human brain produce so much neuromelanin that you can visualise it with the naked eye. As you can see in the image below, the Parkinsonian brain has less dark pigmented cells (in the substantia nigra region of the midbrain). As dopamine neurons – which are affected in Parkinson’s – are lost, so too is the dark pigment of neuromelanin.
 The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
The finding that Parkin may be boosting neuromelanin formation could be supported by postmortem analyses. Several autopsy studies have reported less neuromelanin in the surviving dopamine neurons in the substantia nigra of individuals who were diagnosed with Parkin-associated Parkinson’s (Click here and here to read more about this).
The scientist behind the new bioRxiv research conclude their study by suggesting that their data expands the known functions of Parkin protein, raises concerns about the loss of function of this protein in the ageing human brain, and highlight the possibility of neuroprotective Parkin-based therapies for the future treatment of Parkinson’s.
|
# # RECAP #2: New research finds that Parkin protein exhibits reduced function in the human brain with age. This process is a result of oxidative alterations to the structure of the protein. The effect appears to be specific to the brain and to humans. The study also indicates that Parkin can function as an antioxidant reducing levels of oxidation. # # |
So if people have a genetic variant in their Parkin gene, this might make them more vulnerable to Parkinson’s via oxidative changes?
That is what the new data is suggesting, but it is important to remember that this is new data. It has not gone through the peer review process, and needs to be independently replicated before we get to carried away with speculations as to what it could all mean.
It is interesting though that Parkin-associated Parkinson’s is generally viewed as a more substantia nigra-focused form of Parkinson’s.
As we discussed above, individuals affected by this condition typically do not lose their sense of smell, which is the situation in many other forms of Parkinson’s (we have discussed this idea in previous SoPD post – click here to read more). In addition, dopamine neurons are extremely active and prone to oxidative stress (we have also explored this in previous SoPD posts – click here for an example). So in this context, this new research is very interesting and could explain the ‘young-onset’ aspect of Parkin-associated Parkinson’s.
Has anyone else ever found similar results?
So, I was recently going through the poster abstracts for the up-coming 2021 Alzheimer’s & Parkinson’s Diseases Conference (because that’s what life in COVID lockdown does to you) when I noticed this poster abstract title:
Poster number: P439 /#1494
Topic: Theme C: α-Synucleinopathies / C1.b. Disease Mechanisms, Pathophysiology: LRKK2, parkin, PINK1, DJ-1
Title: OXIDIZED PARKIN, ITS MISFOLDING AND AGGREGATION VIA THE FORMATION OF ABERRANT INTRA-AND INTERMOLECULAR DISULFIDE BONDS PRODUCE CELLULAR TOXICITY
Authors: A. Esmaeili, M. Duennwald (Western University, Pathology, London, Canada)
Now I have not seen their data, but in the abstract for this presentation, the researchers “hypothesized that oxidative stress damages Parkin resulting in its misfolding and altered cellular localization, which contributed to UPS and autophagy dysfunction and thus to PD pathogenesis”. They then test this idea and they results lead them to conclude “that alteration of Parkin solubility induced by various extrinsic and intrinsic stressors provides a mechanism for Parkin misfolding and dysfunction may be relevant to the pathogenesis of sporadic PD”.
And there have been other reports indicating that oxidative changes can affect Parkin function (Click here to read an example), but none have analysed the situation to the depth that the new bioRxiv manuscript has.
Interesting. So what can be done? Are there efforts to restore or increase levels of Parkin protein in people with Parkinson’s?
It’s a good question and the answer is: yes.
As we discussed in the recent “Road ahead: 2021” post, there are considerable research efforts being made to boost levels of Parkin.
One approach that we are watching closely here at the SoPD is being developed by a South Korean biotech firm called Cellivery Therapeutics.
 This company is developing “improved cell-permeable Parkin protein” (iCP-Parkin). It is basically the Parkin protein with a few additional attachments that allows it to pass easily through cell membranes.
This company is developing “improved cell-permeable Parkin protein” (iCP-Parkin). It is basically the Parkin protein with a few additional attachments that allows it to pass easily through cell membranes.
Last year the company published this report on their progress:
 Title: Intracellular delivery of Parkin rescues neurons from accumulation of damaged mitochondria and pathological α-synuclein
Title: Intracellular delivery of Parkin rescues neurons from accumulation of damaged mitochondria and pathological α-synuclein
Authors: Chung E, Choi Y, Park J, Nah W, Park J, Jung Y, Lee J, Lee H, Park S, Hwang S, Kim S, Lee J, Min D, Jo J, Kang S, Jung M, Lee PH, Ruley HE & Jo D
Journal: Science Advances, 29 Apr 2020:6, 18, eaba1193
PMID: 32494688 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers found that iCP-PARKIN treatment significantly reduced levels of alpha synuclein protein aggregation (by more than 80%) in cells that had been treated with neurotoxins. It should also be noted that treating cells with just PARKIN protein (a control) had no effect – due to the protein not entering the cells.
In multiple Parkinson’s models, the iCP-PARKIN treatment improved the motor complications and survival of the dopamine neurons (compared to control treated animals).
The researchers concluded their study by saying that their new therapeutic agent has “the desired activity, specificity, and bioavailability” required for a condition like Parkinson’s, and that their results were consistent across multiple models (using different mechanisms of pathology).
In addition to Cellivery, there are other biotech firms exploring novel methods of boosting Parkin. Vincere bioscience (Vincere comes from the Latin for “To win“) was awarded a grant from the Michael J Fox Foundation in 2019 to accelerate a Parkin activator program (Source).
 In addition, another biotech company called Progenra, received a research grant in 2020 from the Michael J Fox Foundation to accelerate their Parkin activator program.
In addition, another biotech company called Progenra, received a research grant in 2020 from the Michael J Fox Foundation to accelerate their Parkin activator program.
 Here at the SoPD, we are hoping to see a lot more research focused on Parkin boosting agents in 2021.
Here at the SoPD, we are hoping to see a lot more research focused on Parkin boosting agents in 2021.
So what does it all mean?
Every now and then you read a research report and you get really excited.
You come to the end of it, feeling a little breathless and thinking that this is an important piece of the puzzle. That was how I first felt last year when I read the bioRxiv manuscript discussed in today’s post. I was excited by the implications of it and eager to see the peer-reviewer comments and (hopefully) constructive feedback. I was reluctant to write a post about it until it has been properly published, but in the end I just thought that this was too interesting.
I mean, the reported effect is apparently specific to the human brain and to this Parkinson’s-associated protein.
Previously, the majority of Parkin research has centred around its role in a process called mitophagy – the proteins role in the removal of old mitochondria (the power stations of cells). But this function in the context of Parkinson’s has been questioned recently with the discovery that the loss of Parkin does not really affect basal levels of mitophagy (Click here to read more about this). So it is interesting to see new functions for Parkin, particularly if they have more relevance to Parkinson’s (as in the case of oxidation).
Obviously, we need independent replication and a broader analysis. As far as I can determine in addition to Parkin, the other examined proteins in the study were DJ-1, α-synuclein, cytosolic glyoxalase-1, peroxiredoxin-1 and -3, and calnexin – none of which were affected in the same way as Parkin. It would be interesting to see if this age-dependent solubility phenomenon affects other proteins.
But if the result is independently replicated, it would represent a significant development in our understanding of the underlying biology of this particularly form of Parkinson’s and it may have implications for how we might be able to better treat the condition as a whole. And who knows, maybe it will have broader implications for aging in general.
(which would be welcome relief for sissies like me)
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from Club-cleo


Do turquoise killifish (N. furzeri) have neuromelanin?
LikeLike
Hi Rhyothemis,
Interesting question.
Answer: ¯\_(ツ)_/¯
We previously looked at killifish in regards to their alpha synuclein (https://scienceofparkinsons.com/2019/03/03/human/), but I’m not sure the really live long enough to build up much neuromelanin. Will do some digging around.
Kind regards,
Simon
LikeLiked by 2 people