|
# # # # Drug repurposing represents a means of rapidly testing and bring novel therapies to the patient. By testing clinically available drugs – that have well characterised safety records in a particular medical condition – one can determine if a certain biological pathway is playing an influential role in another disease. A good example of this is work currently being done by researchers at the University of Iowa with a drug called terazosin. Terazosin is a treatment used for enlarged prostate issues and high blood pressure, but recent epidemiological data and preclinical work indicates that it may also be useful for Parkinson’s. Recently the team in Iowa published a report on a small pilot clinical study evaluating the agent in a group of people with Parkinson’s. In today’s post, we will look at what terazosin does, discuss what the preclinical and epidemiological research suggests, review the results of the pilot study, and discuss what could happen next. # # # # |
 Source: Worldtravelguide
Source: Worldtravelguide
There has been a lot of important Parkinson’s research conducted in the state of Iowa.
For example, in addition to producing a quarter of the USA’s corn and 1/3 of America’s pork, Iowa is also the home to a large family known to researchers as the ‘Iowa kindred‘ or ‘Spellman-Muenter kindred‘.
First described by Spellman in 1962, the Iowa kindred has a long history in which generations of the family have been inflicted with severe parkinsonisms (the symptoms/features of PD). In the family tree below, the black diamonds represent individuals with Parkinsonisms:
 Source: Researchgate
Source: Researchgate
In 2003, researchers discovered that this family was carrying a multiplication of their alpha synuclein gene:
 Title: alpha-Synuclein locus triplication causes Parkinson’s disease.
Title: alpha-Synuclein locus triplication causes Parkinson’s disease.
Authors: Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K.
Journal: Science. 2003 Oct 31;302(5646):841.
PMID: 14593171
The publication of this report was an important moment in Parkinson’s research history (Click here to read a SoPD post about the early history of alpha synuclein).
More recently, some researchers at the University of Iowa are hoping to continue this legacy of important Parkinson’s research by investigating the potential of a clinically available drug to slow the progression of Parkinson’s.
 Source: Youtube
Source: Youtube
Which drug are they investigating?
This is Prof Michael Welsh of the University of Iowa:
 Source: Sanford
Source: Sanford
His area of interest in research is primarily focused on the cystic fibrosis. Cystic fibrosis is a genetic/inherited condition in which the lungs and digestive system become clogged with a lining of thick mucus.
But Prof Welsh and some of his research colleagues have recently been drawn into Parkinson’s research.
 Prof Welsh (2nd from the right) & colleagues. Source: Dailyiowan
Prof Welsh (2nd from the right) & colleagues. Source: Dailyiowan
They have been drawn in by some interesting observations surrounding a drug called terazosin.
What is terazosin?
Terazosin is a drug that is used to treat high blood pressure (hypertension) and it is also used in men to treat the symptoms of an enlarged prostate (a condition called benign prostatic hyperplasia).
 Terazosin. Source: Wikipedia
Terazosin. Source: Wikipedia
It is a widely used agent with a long and safe clinical history, and it has been well characterised in terms of its mechanism of action.
Specifically, it is an α1-adrenergic receptor antagonist.
Hold up! What on Earth does any of that mean? What is a receptor antagonist?
On the surface of a cell, there are lots of small molecules (called receptors) which act as switches for certain biological processes to be initiated. Receptors will wait for a particular protein to come along and bind to them. By binding to the receptor, the protein will either activate it or alternatively block/inhibit it (not allowing the biological process to be initiated).
The activators are called agonists, while the blockers are antagonists.
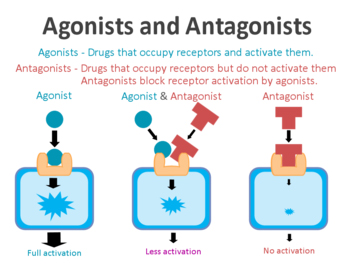
Agonist vs antagonist. Source: Psychonautwiki
Now, terazosin is an antagonist of the α1-adrenergic receptor, meaning that it specifically targets and blocks the α1-adrenergic receptor. And yes, I know what your next question is going to be:
What is the α1-adrenergic receptor?
The α1-adrenergic receptor is a receptor for the neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). There are 3 different subtypes of α1-adrenergic receptor: α1A-, α1B-, and α1D– (don’t ask me what happen to α1C).
The α1-adrenergic receptors are primarily involved with smooth muscle contraction. By binding to and blocking α1-adrenergic receptors, terazosin reduces peripheral vascular resistance (meaning, it lowers blood pressure).
|
# RECAP #1: Terazosin is a widely used treatment for high blood pressure (hypertension) and enlarged prostates It functions by blocking the α1-adrenergic receptor, but new research indicates that it does more than that. # |
Interesting, but what do α1-adrenergic receptors have to do with Parkinson’s?
There has been a lot of research on the adrenergic receptor antagonists in Parkinson’s, but this is not the focus of our discussion today.
What do you mean?
Well, recently, researchers have discovered that terazosin has another action in the body: it activates phosphoglycerate kinase 1.
What is phosphoglycerate kinase 1?
Phosphoglycerate kinase 1 is an enzyme that catalyzes the formation of ATP.
What does that mean? What is ATP?
The fact that you are reading this, means that your body is producing a lot of ATP.
It is the fuel for your cells. It attaches to various proteins and ‘powers’ their function.
You may remember from high school biology class that there are tiny bean-shaped objects within the cells of your body called mitochondria. They are the power house of each cell. They help to keep the lights on. Without them, the party is over and the cell dies.

Mitochondria and their location in the cell. Source: NCBI
Mitochondria are very efficient at converting nutrients from food into Adenosine Triphosphate (or ATP). And we need HUGE amounts of ATP in order to do everything we do at any moment of the day.
FUN FACT: The average human body produces/recycles its own weight in ATP every day!
The production of ATP is a multi-step cycle and one of the first actors in that process is Phosphoglycerate kinase 1 (in a step called glycolysis).
And recently researchers have identified terazosin as a molecule that can activate Phosphoglycerate kinase 1.
A couple of years ago, this research report was published:
 Title: Terazosin activates Pgk1 and Hsp90 to promote stress resistance.
Title: Terazosin activates Pgk1 and Hsp90 to promote stress resistance.
Authors: Chen X, Zhao C, Li X, Wang T, Li Y, Cao C, Ding Y, Dong M, Finci L, Wang JH, Li X, Liu L.
Journal: Nat Chem Biol. 2015 Jan;11(1):19-25.
PMID: 25383758 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers conducted a drug screening experiment to identify clinically available drugs that can reduce cell death (apoptosis). They found that terazosin was a molecule of interest and they next went looking for how it was havng this beneficial effect.
Through a series of experiments, they found that it binds to and activates the enzymatic activity of Phosphoglycerate kinase 1. Interestingly, this activation led to enhanced activity of the heat shock protein/chaperone Hsp90, which plays an important role in cellular homeostasis and promotes multi-stress resistance responses when a cell is damaged or under stress. In cell cultures, they found that terazosin enhanced Phosphoglycerate kinase 1 activity (increasing ATP levels), which blocked cell death when the cells were exposed to toxins.
This result suggested to the investigators that terazosin may have neuroprotective properties, and they wanted to test this idea in models of Parkinson’s.
The scientists behind that study collaborated with the researchers in Iowa to do this, and that effort led to the publication of this report:
 Title: Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases.
Title: Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases.
Authors: Cai R, Zhang Y, Simmering JE, Schultz JL, Li Y, Fernandez-Carasa I, Consiglio A, Raya A, Polgreen PM, Narayanan NS, Yuan Y, Chen Z, Su W, Han Y, Zhao C, Gao L, Ji X, Welsh MJ, Liu L.
Journal: J Clin Invest. 2019 Oct 1;129(10):4539-4549.
PMID: 31524631 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers investigated the properties of terazosin in various models of Parkinson’s.
The researchers started their studies by examining what terazosin does in the brains of mice. They found that terazosin increased the levels of pyruvate – which is the product of glycolysis – and ATP in various regions of the brain, including the substantia nigra (which is a region of the brain severely affected in Parkinson’s).
This result suggested not only that terazosin enters the brain, but also increases ATP production.
 Source: Pinterest
Source: Pinterest
Next the researchers asked if terazosin treatment could protect mice from the neurotoxin MPTP, which kills dopamine neurons in the substantia nigra and induces a Parkinson’s-like state in the animals. They reported that 7 days after the administration of MPTP to the mice, pyruvate and ATP levels dropped significantly, but terazosin treatment prevented this reduction. The drug also reduced the loss of dopamine neurons – even when the researchers delayed initiation of treatment until 7 days post MPTP delivery (a rather remarkable effect!).
They next tested terazosin in two additional neurotoxin models of Parkinson’s (6-OHDA in rats and rotenone in flies), and the drug was found to be neuroprotective across the models. When they reduced levels of Phosphoglycerate kinase 1 in flies, the researchers found that the neuroprotective effect of terazosin was lost. This last experiment suggests that the beneficial effects of the drug were coming primarily from the Phosphoglycerate kinase 1 actions of terazosin, rather than the blocking of α1-adrenergic receptors.
 Source: Ecolab
Source: Ecolab
Next the scientists tested terazosin in genetic models of Parkinson’s. About 10-15% of cases of Parkinson’s are associated with a genetic variation in regions of DNA that increases the risk of developing the condition. One of these regions of DNA is referred to as PINK1. Errors in PINK1 can result in an early onset (around the age of 30 years old) form of Parkinson’s (Click here to read a previous SoPD post on PINK1).
Flies with PINK1 mutations exhibited wing posture defects and decreased ATP levels in the brain. The researchers reported that treating PINK1 flies with terazosin partially reversed these abnormalities.
And the investigators didn’t stop there (seriously, this was a “kitchen sink” report, these guys tested everything!). They also tested terazosin on flies with LRRK2 genetic mutations. Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’) – also known as ‘Dardarin‘ (from the Basque word “dardara” which means “trembling”) is another region of DNA (or gene) where variations have been associated with increased risk of developing Parkinson’s (Click here to read a previous SoPD post about LRRK2). Flies with mutated LRRK2 genes have motor problems, and guess what? When the researchers tested terazosin on flies with LRRK2 genetic mutations, they observed a partial improvement in those motor problems (it’s becoming a bit repetitive, yeah?).
The researchers next tested terazosin on a genetically engineered mouse that produced very high levels of human alpha synuclein protein, and they reported that the terazosin-treated mice had less alpha synuclein in their brains.
 Neurons growing in petri dishes. Source: Salk
Neurons growing in petri dishes. Source: Salk
And finally, they tested terazosin on human cells in culture. These cells had been collected from people with LRRK2-associated Parkinson’s, and converted into neurons. As the neurons grew in culture, approximately 60% of the LRRK2-mutated neurons started accumulating alpha synuclein protein compared with just 15% of the neurons from healthy individuals. And (you guessed it) terazosin reduced the percentage of LRRK2-mutated neurons with accumulated alpha synuclein.
Basically, every PD model that the researchers tested terazosin on suggested the drug has beneficial effects.
It all sounds almost too good to be true, right?…
…but here’s the thing:
Given that terazosin is a commonly used drug, and has been in clinical use for a long time now (it was patented in 1975 and came into medical use in 1985), the researchers decided to analyse a large medical database to determine if taking this drug reduced the incidence of Parkinson’s.
Using the IBM Watson/Truven database, the investigators found that terazosin (or similar drugs) treatment reduced the risk of having any of the 79 Parkinson’s-related diagnostic codes in your medical file by 20% – relative risk = 0.78 (95% CI: 0.74–0.82).
What’s more, when they looked at the Michael J Fox Foundation Parkinson’s Progression Markers Initiative database, the researchers found that individuals with Parkinson’s being treated with terazosin had a reduced rate of progression on the motor symptoms (although it has to be said here that the number of individuals involved in this analysis was very small).
Either way, the result is very intriguing and reminiscent of the beta blocker/beta agonist report in 2017 (Click here to read a SoPD post about that research).
The report does not explain how enhanced phosphoglycerate kinase 1 activation could be slowing neurodegeneration or progression in Parkinson’s, but it may open up a new neuroprotective mechanism for future PD research to explore.
|
# # RECAP #2: Recent research reported that terazosin also functions as a phosphoglycerate kinase 1 activator, and has neuroprotective properties. Experiments in multiple preclinical models of Parkinson’s found that terazosin could be an interesting drug for repurposing. In addition, epidemiological investigations provide supportive data. # # |
Has this neuroprotective effect of terazosin ever been reported before?
Actually, it sort of has.
Back in 2000, this report was published:
 Title: Overexpression of the alpha1B-adrenergic receptor causes apoptotic neurodegeneration: multiple system atrophy.
Title: Overexpression of the alpha1B-adrenergic receptor causes apoptotic neurodegeneration: multiple system atrophy.
Authors: Zuscik MJ, Sands S, Ross SA, Waugh DJ, Gaivin RJ, Morilak D, Perez DM.
Journal: Nat Med. 2000 Dec;6(12):1388-94.
PMID: 11100125
In this study, the researchers genetically engineered mice that produce high levels of α1B-adrenergic receptor, which developed Parkinson’s-like hindlimb motor problems and displayed neurodegeneration that increased with age.
The investigators found that terazosin partially rescued these mice.
And this initial study was followed up by this report:
 Title: Mice expressing the alpha(1B)-adrenergic receptor induces a synucleinopathy with excessive tyrosine nitration but decreased phosphorylation.
Title: Mice expressing the alpha(1B)-adrenergic receptor induces a synucleinopathy with excessive tyrosine nitration but decreased phosphorylation.
Authors: Papay R, Zuscik MJ, Ross SA, Yun J, McCune DF, Gonzalez-Cabrera P, Gaivin R, Drazba J, Perez DM.
Journal: J Neurochem. 2002 Nov;83(3):623-34.
PMID: 12390524 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers further investigated their genetically engineered mice that produce high levels of α1B-adrenergic receptor, and they found that these mice accumulate alpha synuclein over time in brain cells. Interestingly, long‐term treatment with terazosin, not only resulted in protection against the behaviour symptoms and neurodegeneration (as in the first study), but also reduced the accumulation of alpha synuclein.
Interesting. What about the epidemiological data? Has anyone replicated similar results?
There have been several very recent studies looking at this.
Most of the published studies have found a lower incidence of Parkinson’s in individuals who were previously treated with terazosin (compared with use of another α1-adrenergic receptor antagonist, tamsulosin, which does not activate phosphoglycerate kinase 1 like terazosin).
In April 2021, this report was published by the researchers from Iowa:
 Title: Association of Glycolysis-Enhancing α-1 Blockers With Risk of Developing Parkinson Disease.
Title: Association of Glycolysis-Enhancing α-1 Blockers With Risk of Developing Parkinson Disease.
Authors: Simmering JE, Welsh MJ, Liu L, Narayanan NS, Pottegård A.
Journal: JAMA Neurol. 2021 Apr 1;78(4):407-413.
PMID: 33523098 (This report is OPEN ACCESS if you would like to read it)
In this study, they used medical data collected from Danish nationwide health registries and the Truven Health Analytics MarketScan database, and they found that the use of terazosin (and similar drugs like doxazosin & alfuzosin) was associated with a 12% to 37% decrease in Parkinson’s risk (compared with use of tamsulosin).
They concluded their study by saying that “it may be important to identify people with impaired energy metabolism or those who might benefit from glycolysis-enhancing drugs. As such, future investigations are needed to identify whether a specific subset of patients are more likely to benefit from treatment.“.
But then in June 2021, an independent research group at Cerevel Therapeutics published this report:
 Title: Parkinson disease among patients treated for benign prostatic hyperplasia with α1 adrenergic receptor antagonists.
Title: Parkinson disease among patients treated for benign prostatic hyperplasia with α1 adrenergic receptor antagonists.
Auhors: Sasane R, Bartels A, Field M, Sierra MI, Duvvuri S, Gray DL, Pin SS, Renger JJ, Stone DJ.
Journal: J Clin Invest. 2021 Jun 1;131(11):e145112.
PMID: 33822767 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers found that terazosin/alfuzosin/doxazosin use did not alter Parkinson’s risk from matched controls.
They used medical data from the US-based Optum Research Database (collected between January 1st 2010 to December 31st 2019) and they showed that the incidence of Parkinson’s in people treated with tamsulosin was significantly higher than in terazosin/alfuzosin/doxazosin users and matched controls.
But then – just one month later – this third study was published by another independent research group:
 Title: Exposure to Phosphoglycerate Kinase 1 Activators and Incidence of Parkinson’s Disease.
Title: Exposure to Phosphoglycerate Kinase 1 Activators and Incidence of Parkinson’s Disease.
Authors: Gros P, Wang X, Guan J, Lang AE, Austin PC, Welk B, Visanji NP, Marras C.
Journal: Mov Disord. 2021 Oct;36(10):2419-2425.
PMID: 34241922
In this study, the researchers used healthcare administrative data from Ontario (Canada), of 265,745 men older than 66 years and they found that the risk of Parkinson’s for those taking terazosin/doxazosin/alfuzosin was significantly less compared to men taking tamsulosin.
So while a little mixed, the medical database data suggests that terazosin use is having an influence in reducing the risk of developing Parkinson’s.
Has terazosin ever been clinically tested in Parkinson’s?
Yes.
The guy in the middle of the picture below is Dr Jordan Schultz and the chap on the far right is Dr. Nandakumar Narayanan:
 Prof Welsh (2nd from the right). Source: Dailyiowan
Prof Welsh (2nd from the right). Source: Dailyiowan
And working with Prof Welsh at the University of Iowa, they have very recently published this report with fellow collaborators presenting the results of a small pilot study:
 Title: A pilot to assess target engagement of terazosin in Parkinson’s disease.
Title: A pilot to assess target engagement of terazosin in Parkinson’s disease.
Authors: Schultz JL, Brinker AN, Xu J, Ernst SE, Tayyari F, Rauckhorst AJ, Liu L, Uc EY, Taylor EB, Simmering JE, Magnotta VA, Welsh MJ, Narayanan NS.
Journal: Parkinsonism Relat Disord. 2021 Nov 27;94:79-83.
PMID: 34894470
In this study, the researchers conducted a 12-week pilot study to assess the safety and tolerability of terazosin in Parkinson’s. The TZ-PD study (I’ll let you work out that acronym) was a single center, randomised, double-blind, placebo-controlled study and the investigators used 31P-MRS imaging to explore ATP levels in the brain.
What is 31P-MRS imaging?
31P-MRS (or phosphorus magnetic resonance spectroscopy) imaging is a non-invasive imaging technique that allows researchers to get a measure of energy metabolism in a particular region of the body in a living person.
Specifically, 31P-MRS can be used to measure increases (or decreases) in ATP levels in the brain.
31P-MRS deserves a post all of its own, but for those interested in learning more about it, this video provides an excellent overview of the technology:
The researchers recruited 13 people with Parkinson’s (Hoehn-Yahr Stage I-III) and randomly assign them 1:1 to either terazosin or placebo treatment for 12 weeks (Click here to read more about the details of this study).
After 12 weeks of treatment (with either terazosin or placebo), the researchers found that levels of ATP in the brain increased (compared to baseline) in 4 of the 5 people taking terazosin and 2 of the 5 people taking placebo. Critically, the increase in ATP levels was higher for those taking terazosin. And these results were replicated in blood samples, suggesting to the investigators that terazosin is engaging with its target and changing ATP levels in the brain (and blood).
The researchers also found that terazosin was safe and well tolerated by the participants in the treatment group. It is a drug that is used for the treatment of high blood pressure, so common side effects of the drug were mild dizziness/lightheadedness (3 participants eventually pulled out of the study as a result of this).
Did they see any change in Parkinson’s progression?
This was a very small and short study (just 12 weeks treatment), so it was difficult to determine any disease modifying effects. But the goal here was to see if the drug is actually safe in people with Parkinson’s and if it was having the desired effect on ATP levels in the brain.
The researchers believed that based on these results gained larger and longer investigations are warranted to test the disease-modifying potential of terazosin.
Interesting, so what happens next?
The research team in Iowa are currently planning those larger studies, but they are also conducting another small study to further explore target engagement of terazosin (Click here to read more about that study). The Michael J Fox Foundation is supporting that work and the goal is to build up as much data as they can to help guide the design of the future studies investigating the progression limiting potential of terazosin.
There is also a new study looking at terazosin in individuals with Lewy body dementia. This is another pilot study, but it is looking to recruit 40 participants for for 15 weeks who will be treated with placebo or two different doses of terazosin (Click here to read more about the TZ-DLB study).
So what does it all mean?
There’s a lot of interesting stuff in Iowa – the State Capitol’s Law Library is one example:
 How do they get any work done? Source: Housebeautiful
How do they get any work done? Source: Housebeautiful
And a lot of interesting Parkinson’s research coming out of the state.
The discovery of a novel mechanism of action for a clinically used therapy provides the opportunity for drug repurposing. And drug repurposing is just the first step in the process of bringing new treatment options to patients.
Subsequent steps involve replication studies, conducted by independent research groups across related conditions (and we are already seeing this with terazosin – there is a pilot study at Cedars-Sinai Medical Center in Los Angeles exploring terazosin in individuals with REM Behavior Sleep Disorder (or RBD – Click here to read more about this). RBD is a sleep disorder that results in people to ‘acting out’ their dreams, and it is believed to be a risk factor for developing Parkinson’s.
If all of this research produces interesting results we will then hopefully see additional positive outcomes of drug repurposing efforts with appearance of biotech companies developing novel brain penetrant phosphoglycerate kinase 1 activators that do not have the blood pressure side effects of terazosin. We have seen this biotech trend already with the repurposing of the diabetes drug exenatide for Parkinson’s in the case of the biotech companies like Neuraly and Peptron (Click here to read a SoPD post on this topic).
So fingers crossed that this repurposing research in Iowa will lead to such positive outcomes. It will be interesting to watch how this particular situation develops in 2022.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from Medicinehow




Is alfuzozin the same as terazozin? Your website explains what is complex in simple terms
LikeLike
I tried to contact at the university of Iowa but I never heard back from them about this research
LikeLike