|
Alpha synuclein is a protein that is closely associated with Parkinson’s. But exactly if and how it is connected to the neurodegenerative process underlying the condition, remains unclear. Last week researchers reported that removing a particular form of alpha synuclein in mice results in a very early onset appearance of characteristics that closely resemble the features of Parkinson’s that we observe in humans. This finding has caused some excitement in the research community, as not only does this tell us more about the alpha synuclein protein, but it may also provide us with a useful, more disease-relevant mouse model for testing therapies. In today’s post, we will discuss what alpha synuclein is, explain which form of the protein was disrupted in this mouse model, review the results of the new study, and look at how tetramer stablising drugs could be a new area of PD therapeutics.
|
 The 337 metre (1,106 ft) long USS Gerald R. Ford. Source: Wikipedia
The 337 metre (1,106 ft) long USS Gerald R. Ford. Source: Wikipedia
Imagine you and I are standing in front of the world’s largest aircraft carrier, the USS Gerald R. Ford.
It is a VAST warship – measuring in at 337 metres (1,106 ft) in length, 76 metres (250 feet) in height – and it is a wonder of engineering composed of over a billion individual components.
And as we are standing there, gazing up at this amazing machine, I turn to you and put a nut & bolt into the palm of your hand.
 A nut and bolt. Source: Atechleader
A nut and bolt. Source: Atechleader
You look down at it for a moment, then turn to me, puzzled.
And that is when I say: “I would like you to find (without aid/instructions) where on this ship versions of this particular type of nut and bolt live, and try to determine exactly what functions they have“.
Where would you even start?
What tools would you use for the job? Considering the size and complexity of the vessel, would you simply give up before even starting?
It sounds like a ridiculously daunting task, but this is in effect what neurobiologists are trying to do with their study of the brain. They start with a protein – one of the functional pieces of machinery inside each cell of our body – and then try to determine where in the brain it lives (the easy part) and what it does exactly (the REALLY hard part – most proteins have multiple functions and different configurations).
A good example of this is the Parkinson’s-associated protein alpha synuclein:
Alpha synuclein. Source: Wikipedia
Alpha synuclein is one of the most abundant proteins in our brains – making up about 1% of all the proteins floating around in each neuron in your head – and it is a very well studied protein (with over 9700 research reports listed on the Pubmed search engine with the key words ‘alpha synuclein’).
But here’s the thing: we are not entirely clear on what alpha synuclein actually does inside the cell.
Que?
In fact, biologists are not even sure about what the ‘native’ form of alpha synuclein is!
What do you mean?
When alpha synuclein protein is produced by a cell, it normally referred as an ‘unfolded protein’, in that is does not really have a defined structure. When it is first produced, alpha synuclein will look something like this:
Alpha synuclein. Source: Wikipedia
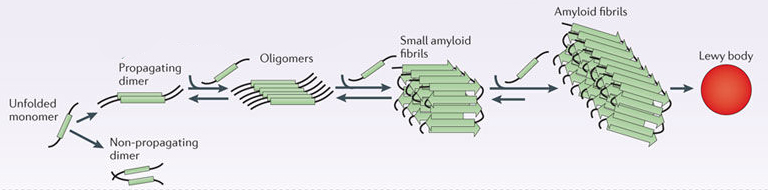
In this form, alpha synuclein is considered a monomer – which is a single molecule, just one copy of the protein. It is capable of binding to other molecules, and when it binds to other alpha synuclein proteins, they form what is called an oligomer (a collection of monomers). And these oligomers can have different structures.
In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of Monomers, oligomers and fibrils. Source: Brain
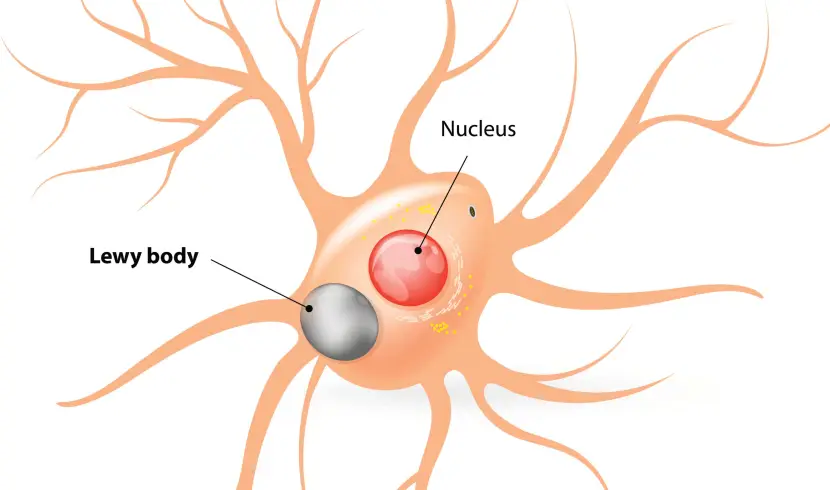
And it is believed that these oligomer and fibril forms of alpha synuclein protein may go on to produce the Lewy bodies that characterise the Parkinsonian brain.
Parkinson’s associated alpha synuclein. Source: Nature
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
A cartoon of a neuron, with the Lewy body (indicated) within the cell body. Source: Alzheimer’s news
But with all of that said, we still come back to my statement above:
We do not know what the native form of alpha synuclein is
But what does that mean?
The native form of a protein is its properly folded and/or assembled state. It is the version of the protein that is ‘operative and functional’.
But in the case of alpha synuclein, we are not sure if it is a monomer or oligomer form of the protein that is actually functional (or perhaps the answer is both). And this has resulted in a lot of research and great deal of academic debate about the true nature of alpha synuclein.
And this ‘native’ debate kicked off again last week with the publication of this research report:
Title: Abrogating Native α-Synuclein Tetramers in Mice Causes a L-DOPA-Responsive Motor Syndrome Closely Resembling Parkinson’s Disease
Authors: Nuber S, Rajsombath M, Minakaki G, Winkler J, Müller CP, Ericsson M, Caldarone B, Dettmer U, Selkoe DJ.
Journal: Neuron. 2018 Oct 10;100(1):75-90.e5.
PMID: 30308173
In this study, the researchers found that disrupting the ability of alpha synuclein to form tetramers in mice resulted in those mice developing features very similar to Parkinson’s.
Hang on a second. What is a ‘tetramer’?
A tetramer (tetra-, “four” + –mer, “parts”) is an oligomer formed from four monomers. In the case of alpha synuclein, four individual monomers of the alpha synuclein protein combine to form a tetramer.
Is this the native state of alpha synuclein?
As I suggested above, the answer to this question is:
We really don’t know.
But the researchers behind this new study, believe that alpha synuclein tetramers have a very important role in the functional state of alpha synuclein. And they have spent quite a bit of time looking into this.
They first reported alpha synuclein tetramers in 2011:
Title: α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation.
Authors: Bartels T, Choi JG, Selkoe DJ.
Journal: Nature. 2011 Aug 14;477(7362):107-10.
PMID: 21841800 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to address the question of the native form of alpha synuclein. Most of the research exploring the function of alpha synuclein at the time required huge amounts of the protein, and to achieve this scientists would use bacteria or genetically engineering cells to pump out vast amounts of the protein. But these high levels may not necessarily reflect what is naturally occurring inside a cell. Such high levels of the protein could be misleading.
So the researchers who conducted this 2011 study isolated and analyzed alpha synuclein protein from both neuronal and non-neuronal cell lines, as well as brain tissue and living human cells. And using multiple methods of analysis, they found that alpha synuclein occurs in cells to a large degree as a tetramer – a structure made up of four individual monomers of alpha synuclein connected together.
Four alpha synuclein monomers forming a tetramer. Source: PNAS
And interestingly, while the alpha synuclein monomers produced at high levels by the traditional “bacteria or genetically engineered cells” approach readily aggregated into fibrils, the tetramers that the researchers had isolated from primary sources did not really aggregate at all.
Based on these results, the investigator proposed that idea that any agent which destabilises the natural tetramer formation of alpha synuclein, could lead to mis-folding of the protein or a build up of monomers which may result in increased levels of protein aggregation similar to that observed in Parkinson’s. They also suggested that small molecules which stabilise these tetramer could be potentially very useful in the treatment of Parkinson’s.
So there was now a new form of alpha synuclein wandering around – in addition to the monomeric version which tends to bind to lipid membranes. The tetrameric form of alpha synuclein joined the great scheme of things:
The different forms of alpha synuclein. Source: Sciencedirect
And a few years after that first report, the researchers extended their initial analysis and published this study:
 Title: Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation.
Title: Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation.
Authors: Dettmer U, Newman AJ, Soldner F, Luth ES, Kim NC, von Saucken VE, Sanderson JB, Jaenisch R, Bartels T, Selkoe D.
Journal: Nat Commun. 2015 Jun 16;6:7314.
PMID: 26076669 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers assessed fresh human brain biopsies (collected from elective surgery for focal epilepsy) for alpha synuclein tetramers. And again, they found that these brain biopsies from non-Parkinsonian individuals contained abundant alpha synuclein tetramers.
Next they questioned whether genetic mutations in the alpha synuclein gene could disrupt these tetramers. Genes are the regions of DNA responsible for providing the instructions for making alpha synuclein protein, and genetic mutations are variations or mistakes in the coding in that region of DNA. Genetic variations in the alpha synuclein gene are associated with increased risk of a person developing Parkinson’s, therefore it is an interesting question.
To address this, the investigators looked for tetramers in the brains of mice that carried human genetic mutations in their alpha synuclein gene. The investigators found that mice with ‘A53T’ alpha synuclein mutation exhibited reduced levels of tetramers in their brains (Click here to read more about the A53T mutation). They also found that mice with another genetic mutation (E46K) in the alpha synuclein gene also had reduced levels of alpha synuclein tetramers in the brain.
And they also found this result (reduced levels of alpha synuclein tetramers) to be true in human cells (with A53T genetic variations) grown in culture.
The interesting thing about these two particular genetic variations is that when you look at the alpha synuclein gene, both the A53T and the E46K mutations lie within the what is called the Amphipathic region (the red area in the image below):
The layout of alpha synuclein DNA with locations of mutations. Source: Mdpi
The amphipathic region of alpha synuclein is the part of the protein that binds to cell membranes and has the ability to remodel them (Click here to read more about this). Within the amphipathic region, there is a KTKEGV repeat (see image above) which is actually responsible for binding to cell membranes. Now, given that this region also contains mutations that reduce the presence of tetramers, the investigators asked if further disruption of this membrane-binding region would reduce the likelihood of tetramers forming.
To address this, they disrupted the KTKEGV repeat with individual and then multiple errors, and they found that this resulted in less tetramers, increased monomers, and induces neurotoxicity (which was characterised by “round inclusions” – sound familiar?!?).
It has to be said here that several independent research labs have also investigated this idea of a tetramer and some have failed to find them (Click here and here to read examples of this research). Those studies have reported that “native alpha synuclein purified from mouse brain consists of a largely unstructured monomer, exhibits no stable tetramer formation, and is prone to aggregation” (Source). Other research groups have been able to find them (Click here for an example), and have even identified particular functions for these tretramers (Click here to read more).
But the researchers who originally identified them repeated their analysis and found that genetic errors in the KTKEGV repeat region of alpha synuclein did appear to disrupt the tetramers ability to hold together:
Title: KTKEGV repeat motifs are key mediators of normal α-synuclein tetramerization: Their mutation causes excess monomers and neurotoxicity.
Authors: Dettmer U, Newman AJ, von Saucken VE, Bartels T, Selkoe D.
Journal: Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):9596-601.
PMID: 26153422 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers found that just one genetic variation in the middle of the KTKEGV repeat was sufficient to reduce the levels of tetramers, but this effect was amplified by making two additional mutations. And this reduction subsequently triggered more severe alpha synuclein aggregation and toxicity in the cells.
But all of this research was conducted in cells, which left the researchers wondering:
What would happen if we did this in mice?
Source: Pinterest
And that gave rise to the study that we are reviewing today:
Title: Abrogating Native α-Synuclein Tetramers in Mice Causes a L-DOPA-Responsive Motor Syndrome Closely Resembling Parkinson’s Disease
Authors: Nuber S, Rajsombath M, Minakaki G, Winkler J, Müller CP, Ericsson M, Caldarone B, Dettmer U, Selkoe DJ.
Journal: Neuron. 2018 Oct 10;100(1):75-90.e5.
PMID: 30308173
The researchers genetically engineered mice with normal human alpha synuclein, or human alpha synuclein with one variation in the KTKEGV repeat region, or human alpha synuclein with three variations in the KTKEGV repeat region. As expected, the investigators found that the mice with the genetic mutations had decreased levels of tetramers in their brains, and increased levels of monomers. And this reduction in tetramers depended on the number of mutations – while just one mutation decreased the number of tetramers (compared to monomers) by 40 percent, three mutation reduced that ratio by more than 90 percent!
But unexpectedly, this severe reduction in tetramers was also associated with a dramatic change in the motor ability of these mice starting at a very early age (from 3 months). In general, the PD research community has not had much success with genetic mouse models of Parkinson’s, and most of them either do not develop motor impairments or do so very slowly (with their appearance starting very late; around 12-18 months of age). But in this study, the mice with three variations in the KTKEGV repeat region, started to display a spontaneous head and body tremor at 3 months of age. And this gradually got worse with age. Over time, the coordination of the mice became affected, demonstrating itself in their limited ability to climb a pole and balance on a rotating rod – both of which got worse with time. At six months of age, the mice with three variations in the KTKEGV repeat region moved around around their home cage a lot less than its counterparts, and when they did they did so with a very stiff gait.
Intriguingly, the researchers found that these motor/movement issues were more robust in male mice than female, which seems to reflect what is seen in humans (a higher ratio of males being affected than females).
These motor/movement issues were also associated with changes in the brains of the mice.
By 6 months of age, very clear aggregation of alpha synuclein protein was observed. In the image below, you can see that the mice with one variations in the KTKEGV repeat region (1K) and three variations in the KTKEGV repeat region (3K) had alpha synuclein (stained in red/brown) accumulating over time (compared to the normal wild-type (WT) control mouse):
 Source: Alzforum
Source: Alzforum
In addition to this build up of aggregated alpha synuclein, the 6 month old mice with three variations in the KTKEGV repeat region had 27% fewer dopamine neurons (when compared with the 6 month old control mice). The loss of dopamine neurons is a cardinal feature of Parkinson’s, and thus the researchers were very excited – they have a mouse that replicates many of the features of the human condition.
These results led the researchers to conclude that continuously inhibiting tetramers leads to excessive levels of monomers which can result in a Parkinson’s-like state in mice. They also proposed that a decrease in the levels of alpha synuclein tetramers compared to monomers may underlie certain genetic forms of the condition.
Thus, agents that stabilise alpha synuclein tetramers may be a useful method of treating Parkinson’s.
Is there any way to stabilise alpha synuclein tetramers?
Not that I am aware of (and I would be very pleased to be corrected on this).
But following this new research report, you can basically bet the house (just a figure of speech!) that there will be different research groups around the world right now madly trying to identify any proteins that display “alpha synuclein tetramer stabilising” properties.
Has anyone ever identified tetramer stablising proteins before?
Yes, they have.
And recently there has been positive news from a very good example of a tetramer stabilising compound.
Approximately, 10-15% of adults over the age of 65 will have cardiac amyloid deposits. These are build ups of aggregated protein around the heart, and they can lead to a condition known as amyloid cardiomyopathy, which is a form of restrictive cardiomyopathy. It occurs when transthyretin (the protein in your blood) fails to stay in its normal tetramer configuration and starts to cluster in amyloid fibrils. This build up ultimately weakens the heart, and if left untreated it can be fatal.
 Source: Slideshare
Source: Slideshare
Until recently, treatment options have been limited.
But there has been a lot of research activity focused on tetramer stabilising compounds, and very recently (like, last month!), the results of a massive Phase III clinical trial of one such drug provided evidence that some of these tetramer stabilising compounds can have beneficial effects:
 Title: Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy
Title: Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy
Authors: Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C; ATTR-ACT Study Investigators.
Journal: N Engl J Med. 2018 Sep 13;379(11):1007-1016.
PMID: 30145929
In this study, the major pharmaceutical company Pfizer tested their transthyretin tetramer stabilising drug Tafamidis on 441 randomly-assigned amyloid cardiomyopathy patients. Tafamidis binds to transthyretin, and prevents its tetramer formation from dissociating. The results of the study suggest that Tafamidis was associated with a lower level of mortality and cardiovascular-related hospitalizations when compared with placebo.
If nothing else, this example provides us with evidence that, yes, tetramer stabilising compounds are possible and can have beneficial effects.
PLEASE NOTE: Just because Tafamidis had a beneficial effect in amyloid cardiomyopathy does not mean that it could have any effect in Parkinson’s. Tafamidis is designed to bind specifically to the protein transthyretin, not alpha synclein. Please do not ask your doctor for this drug (while also citing this website).
What does it all mean?
Determining the ‘native’ state (the functional form) of the protein alpha synuclein would represent a major step forward in our understanding of the biology underlying Parkinson’s. It is, however, very unlikely that it will be a simple answer (if it was, then we would probably be more simple than we are). There may well be multiple ‘native’ states of alpha synuclein and they may have a multitude of different/overlapping functions. But if we can identify states of the protein that are closely associated with the neurodegenerative processes, then we would be making a major leap forward.
Recently, researchers have provided very intriguing evidence that this Parkinson’s-associated protein can form a tetramer formation (made up of four individual alpha synuclein proteins stuck together in a specific shape). In addition, they have presented evidence that continuous disruption of that structure results in the build up of alpha synuclein protein, which in mice is associated with a Parkinson’s-like state (both behaviourally and pathologically).
If these results can be independently replicated and verified, this would be very exciting as it not only enhance our knowleadge of the biology of PD, but it would also provide us with a powerful new model to test many of the drugs being developed for this condition. It would also orient us towards developing alpha synuclein tetramer stabilising compounds as potential future therapeutics. In such a scenerio, one could envisage a potential treatment for Parkinson’s which would combine A.) alpha synuclein tetramer stabilising drugs (which would help maintain the ‘native’ form of alpha synuclein) with B.) an immunotherapy approach (which would clear out the excess, aggregated form of the protein – we have discussed immunotherapy on this website quite regularly, click here to read a recent post on this topic).
As we have discussed, we have multiple immunotherapy approaches currently being clinically tested, now all we need are some alpha synuclein tetramer stabilising compounds.
And if the tetramer results are independently replicated, I don’t think the stabilising compounds will be too far away.
The banner for today’s post was sourced from Greg Dunn (we love his work!)










Hi, and hope you are well – the My Tweets link is sadly not working for me. I get redirected to a ‘search box with a very long number’ which fails to recover anything No sign of a single tweet
all the best,
chris
LikeLike
Hi Chris,
Thanks for your comment, but I’m afraid I can’t help with any technical ‘My Tweet’ issues. Putting material up on this website is about the extent of my technical abilities.
Kind regards,
Simon
LikeLike
Where might mannitol fit in with this if at all? And thank you for your excellent explanation about these new findings. Somehow it helps.
Janet
LikeLike
Hi Janet,
That’s a really interesting idea. It will be interesting to see if Mannitol is in the eventual list of alpha synuclein tetramer stabilising compounds. Glad the website helps.
Kind regards,
Simon
LikeLike
Hi Simon. Thanks for the great post! This, indeed sounds promising, and not a little surprising. However, isn’t a tetramer technically an oligomer? And as such, is it not susceptible to targeting by these immunotherapy agents?
Thanks again. Hope the new job is going well.
LikeLike
Hi Tom,
Thanks for your interesting comment. It is a hard question to answer as the exact target of most of the immunotherapy approaches being developed are unknown (trade secrets). They all claim to be targetting ‘toxic oligomeric’ alpha synuclein, but specifically which part of the oligomer is unknown. Whether this would affect tetrameric alpha synuclein is yet to be determined. I appreciate that this doesn’t answer your question, but I’m not sure if anyone has the answer – it would be an interesting experiment for Prof Selkoe and his team to conduct (if the biotech companies are prepared to provide some of their antibodies).
Kind regards,
Simon
LikeLike