|
Immunotherapy is an experimental treatment that is being tested in Parkinson’s in the hope that it will be able to slow down the progression of the condition. This week the Pharmaceutical company Biogen provided an update regarding their immunotherapy program for Parkinson’s. It involves a drug called BIIB054. In today’s post we will look at what BIIB054 is, how it works, and review the results of Biogen’s first clinical trial with this treatment. |
This week the 2018 American Academy of Neurology ANN Annual Meeting is being held in Los Angeles (California). The meeting is an opportunity each year for researchers to meet and share new discoveries. A lot of neuroscience-focused biotech companies use the meeting to release new clinical trial results.
And this year one result in particular has been rather encouraging.
At 3:30pm on 24th April, the pharmaceutical company Biogen made a presentation entitled “Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose Study of AntiAlpha-Synuclein Antibody BIIB054 in Patients with Parkinson’s Disease,” which provided some of the first insights into the companies immunotherapy program for Parkinson’s.
What is immunotherapy?
Immunotherapy is a method of boosting the body’s immune system to better fight a particular disease.
It involves utilising the immune system of your body, and artificially altering it to target a particular protein/disease-causing agent that is not usually recognised as a pathogen (a disease causing agent).
Immune cells attacking a cancer cell. Source: Lindau-nobel
It is potentially a very powerful method of treating a wide range of medical conditions, and the research on immunotherapy is particularly robust in the field of oncology (‘cancer’). Numerous methods of immunotherapy have been developed for cancer and are currently being tested in the clinic (Click here to read about the many clinical trials now under way).
Many approaches to immunotherapy against cancer. Source: Bloomberg
One of the most promising of these cancer-based immunotherapy approaches is called CART immunotherapy (or chimeric antigen receptor (CAR) T-cell immunotherapy).
This video explains how CART immunotherapy works:
Interesting, but how is immunotherapy being used against Parkinson’s?
One of the big theories about how Parkinson’s progresses involves the idea that a toxic form of the Parkinson’s-associated protein, alpha synuclein, could be being passed from cell to cell.
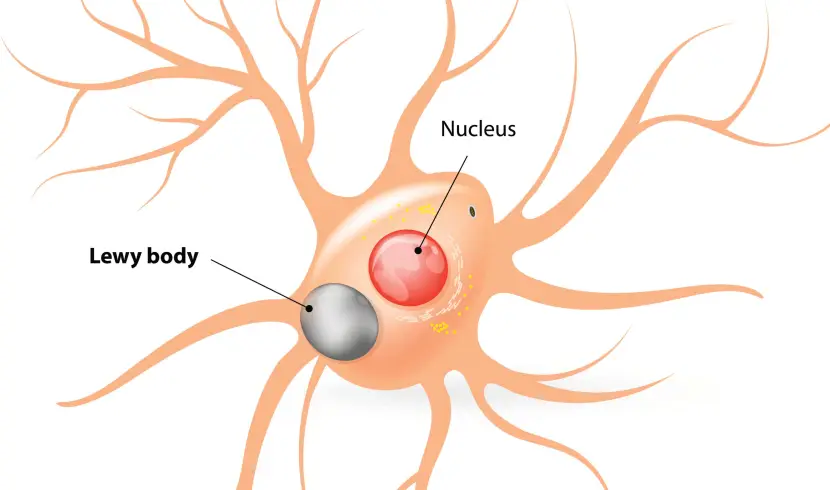
And as this toxic version of alpha synuclein is absorbed by each new healthy cell, it starts causing trouble in that healthy cell and this results in clustering (or aggregation) of protein, which is believed to lead to the appearance of Lewy bodies in those previously healthy cells.
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells – see the image below where alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (stained in brown; indicated by arrows). Source: Wikimedia
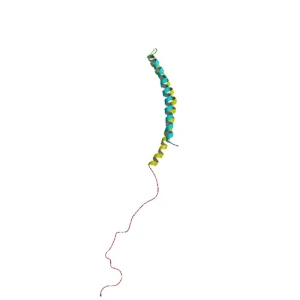
When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure. Alone, it will look like this:
Alpha synuclein. Source: Wikipedia
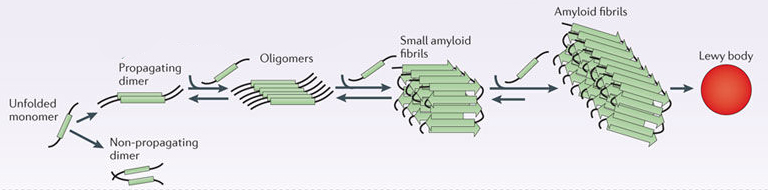
By itself, alpha synuclein is considered a monomer, or a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of Monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein give rise to the aggregations of protein that we refer to as Lewy bodies:
Parkinson’s associated alpha synuclein. Source: Nature
And it also believed that the oligomer and fibril forms of alpha synuclein protein are being passed from cell to cell, and ‘seeding’ protein aggregation in new cells. And this is how the condition may be slowly progressing.
Is there any evidence of this transfer of the alpha synuclein protein?
So back in the 1990s, there were a series of clinical trials of cell transplantation conducted on people with Parkinson’s. The idea was to replace the cells that have been lost to the condition (Click here to read a previous post about cell transplantation). Many of the individuals who were transplanted have now passed away by natural causes and their brains have been examined post-mortem.
One very interesting finding from the analysis of those brains is that some of the cells in the transplants have Lewy bodies in them (up to 10% of transplanted cells in one case – Click here to read the research report on that case).
Above are photos of neurons from the post-mortem brains of people with Parkinson’s that received transplants. White arrows in the images above indicate lewy bodies inside transplanted cells. Source: The Lancet
This finding suggested to researchers that somehow this neurodegenerative condition is being passed on from the Parkinson’s affected brain to the healthy transplanted cells.
And researchers have proposed that the toxic form of the Parkinson’s-associated protein alpha synuclein may be the guilty party in this process (Click here to read more on this idea and the evidence for it).
So how is immunotherapy being applied to Parkinson’s?
One way of dealing with this problem of cell-to-cell transfer of the toxic form of alpha synuclein is to grab it as it is being passed between the cells, and remove it from the body.
This idea has given rise to a series of ongoing clinical trials that are using antibodies which target the toxic form of the alpha synuclein protein.
What are antibodies?
Antibodies are Y-shaped proteins that the immune system naturally and continuously produces to identify anything in the body that is ‘not self’ (that is, not a normally occurring part of you – think of viruses, bacteria, etc).

Monoclonal antibodies. Source: Astrazeneca
Antibodies act like alert flags for the immune system. When antibodies bind to something, they alert the immune system to investigate and potentially remove. Each antibody targets a very specific structure, while ignoring everything else.
In this fashion, antibodies are a very powerful method of removing items from the body that are causing trouble or not wanted.
And researchers have adapted this natural system for Parkinson’s using immunotherapy approaches. Currently, immunotherapy is being tested in Parkinson’s in two ways:
- Active immunisation – this approach involves the body’s immune system being encouraged to target the toxic form of alpha synuclein. The best example of this is a vaccine – a tiny fragment of the troublesome pathogen is injected into the body before the body is attacked, which helps to build up the immune systems resistance to the pathogen (thus preventing the disease from occurring).
- Passive immunisation – this approach involves researchers actually designing antibodies themselves that specifically target a pathogen (such as the toxic form of alpha synuclein, while leaving the normal version of the protein alone). These artificially generated antibodies can then be injected into the body.
Immunotherapy. Source: Acimmune
There are several biotech companies clinically testing immunotherapy approaches for Parkinson’s, including a company called Prothena (Click here to read a previous post on their approach) and an Austrian company called AFFiRiS (Click here to read a previous post on this company).
And which approach is Biogen using against Parkinson’s?
Biogen has taken the second approach (Passive immunisation) with their treatment called BIIB054.
BIIB054 is a monoclonal antibody that has been designed to target the toxic form of alpha synuclein (that is the aggregated form of alpha synuclein, while leaving the monomer form of the protein alone). It was originally developed by a company called Neurimmune, before being licensed to Biogen for clinical testing.
To date, no peer-reviewed data are published about this antibody. As with many of the immunotherapy approaches (AFFiRiS, Prothena, etc), exactly what part of the alpha synuclein protein BIIB054 binds to is unknown – these are secrets that the company’s hold on to very tightly.
While I understand the importance of businesses protecting intellectual property, the lack of published data makes it very difficult to evaluate these immunotherapy approaches.
And what were the new result Biogen presented?
From July 2015 through 2017, Biogen evaluated BIIB054 in 48 healthy volunteers and 18 people with early Parkinson’s (aged 47-75 years, 13 men), at eight sites in the U.S (Click here to read more about the details of this clinical trial).
The primary objective of the study is to evaluate the safety and tolerability of a range of single intravenous doses of BIIB054 (15-45 mg/kg) or placebo. The participants in the study were evaluated for 16 weeks with clinical, brain imaging, electrocardiogram (measuring the heart’s electrical activity), and additional laboratory assessments.
The results indicate that BIIB054 has a blood half-life – the time required for a 50% reduction in the amount of a drug in the blood – of approximately 30 days in Parkinson’s patients. When the results of the healthy volunteers and Parkinson’s subjects were compared, they were found to be very similar.
Most importantly, BIIB054 was reported to be very safe and it was found to be binding to alpha synuclein in the blood, confirming the molecule’s binding to alpha-synuclein. In addition, none of the participants developed an immune reaction (anti-BIIB054 antibodies) to the treatment.
One note of concern was the low amount of BIIB054 actually getting into the brain. The average amount of BIIB054 in the cerebrospinal fluid (which is the fluid that the brain and spinal cord sits in) was just 0.4% of the amount of BIIB054 in the blood. This is a small fraction and suggests that there could be problems with the drug crossing the blood brain barrier. That said, perhaps only a small amount of BIIB054 actually entering the brain is required for it to have a beneficial effect. We will have to wait and see what the Phase II study results suggest.
What happens next?
Based on these results, Biogen has confidently begun a Phase II trial. In December 2017, they started the SPARK study.
This is a 2-year Phase II clinical trial that will test BIIB054 in an estimated group of 311 people with Parkinson’s. It will compare monthly infusions of 3 different doses of BIIB054 (250mg, 1250mg, or 3500mg) and these will be blindly compared to a placebo treated group. Participants who receive the placebo treatment during the first year of the study will be randomly reallocated into one of the active treatment arms in year 2 and will receive BIIB054 every 4 weeks (from week 52 until the end of the study).
The goal of the study will be to:
- Evaluate the safety of multiple doses of BIIB054 over time
- Assess the pharmacodynamic effects of the antibody (or how a drug affects the body)
- Assess the pharmacokinetics (how the body affects the drug) and immunogenicity of BIIB054
The primary outcome measures for the Spark study include adverse events, number of clinical labs, neurological exam, as well as EKG and MRI. Secondary measures include I123 SPECT (or DaTscan) brain imaging assessments, BIIB054 blood serum concentrations, and the percent of participants generating anti-BIIB054 serum antibodies (that is, will the body generate an immune response against the treatment itself?).
Click here to read more about the details of this study.
The Spark trial is being conducted across 17 U.S. sites, and it is set to run until mid-2022.
So what does it all mean?
The first step in ‘curing’ Parkinson’s is to stop the progression of the condition. Numerous approaches are currently being tested in the clinic, with a lot of hope riding on a novel method called ‘immunotherapy’. This “immune system boosting” technique allows us to target specific types of protein that we believe are playing a role in the underlying biology of PD.
This week the pharmaceutical company Biogen presented new results that indicate that their immunotherapy approach – an antibody called BIIB054 – is safe in humans and can now be tested in a larger Phase II clinical trial (which is now underway).
Here at the SoPD we will be watching this and other immunotherapy studies for Parkinson’s very closely (expect regular posts!).
EDITOR’S NOTE: Biogen and Prothena Therapeutics are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. Biogen and Prothena Therapeutics have not requested that this material be produced, nor has the author had any contact with the companies or any associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Wikipedia








Lets hope!!! Iets really something the can cure parkinson. !!
LikeLike