|
Lipids are ‘waxy’ molecules that make up a large proportion of your brain and they play very important roles in normal brain function. For a long time researchers have also been building evidence that lipids may be involved with neurodegenerative conditions as well. Recently, new research was presented that supports this idea (in the case of Parkinson’s at least), as two research groups published data indicating that certain lipids can influence the toxicity of the Parkinson’s associated protein alpha synuclein. One of those research groups was a biotech company called Yumanity, and they are developing drugs that target the enzymes involved with the production of the offending lipids. In today’s post, we will look at what lipids are, what the new research suggests, and discuss some of the issues that will need to be considered in the clinical development of these lipid enzyme inhibitors.
|
 Yummy. Source: Healthline
Yummy. Source: Healthline
Adherence to the ‘Mediterranean diet‘ has been associated with a reduced risk of developing Parkinson’s (Click here and here to read more about this), but no one has ever really explained why.
There has been the suggestion from some corners that this association may be due to the richness of monounsaturated fats in the foods generally included in this diet.
For example, olive oil is rich in monounsaturated fat.
What are monounsaturated fats?
Mmmm, before I answer that we need to have a broader discussion about “what is fat?“.
Fat is one of the three main macronutrients (carbohydrate and protein being the other two) that the body requires for survival.
 Source: Visionpt
Source: Visionpt
Fat serves as a ready source of energy for the body and can also provide insulation against cold temperatures or compression. All fats are derived from combinations of fatty acids (and also glycerol).
What are fatty acids?
A fatty acid is simply a chain of hydrocarbons terminating in a carboxyl group (having a carbonyl and hydroxyl group both linked to a carbon atom). Don’t worry too much about what that means, just understand that fatty acids are basically chains of hydrocarbons that look like this:
 A chain of hydrocarbons ending in a carboxyl group (right). Source: Wikipedia
A chain of hydrocarbons ending in a carboxyl group (right). Source: Wikipedia
Fatty acids come in two forms:
- Saturated
- Unstaturated
In the case of a saturated fat, each carbon molecule in the chain of hydrocarbons is bonded to two other carbons by a single bond. Whereas in the case of a saturated fat, one or more carbon molecule in the chain of hydrocarbons is bonded to another carbon molecule by a double bond. For example:
 Saturated fatty acids vs unsaturated fatty acids. Source: Medium
Saturated fatty acids vs unsaturated fatty acids. Source: Medium
And unsaturated fatty acids can be further divided into:
- Monounsaturated fatty acids (or MUFAs) are simply fatty acids that have a single double bond in the fatty acid chain with all of the remainder carbon atoms being single-bonded.
- Polyunsaturated fatty acids (or PUFAs) are fatty acids that have more than one double bond.
 Source: Medium
Source: Medium
OK, but how might monounsaturated fats be involved with Parkinson’s?
That, dear reader, is the focus of numerous studies in the field of lipidomics.
What is lipidomics?
Lipidomics is the large-scale study of pathways and networks of cellular lipids in biological system.
What are lipids?
Lipids are a large class of molecules of which fatty acids form a small part. On a very basic level, lipids can be defined as molecules that do not interact well with water (and on a more complicated level: lipids are molecules that are soluble in nonpolar solvents).
There are many different types of lipids. And they can come in all sorts of different shapes, for example:
Different types of lipids. Source: Bionumbers
But why are lipids important?
They are important because the outer wall (or membrane) of every cell in your body is made up of lipids (up to 98% of the membrane in some cases). The lipid bilayer of most cell membranes is critical to the survival of organisms. Without lipids, we would be in big trouble.
 A cross section of a cell membrane. Source: Biofoundations
A cross section of a cell membrane. Source: Biofoundations
For more on lipids and cell membranes, watch this video from Khan Academy:
OK, so lipids are important, but how might they be involved with Parkinson’s?
We have known for a long time that a very large portion of your brain is made up of lipids (Click here to read more about this) and we have also known for some time that the Parkinson’s associated protein alpha synuclein associates with lipids in the membranes of cells (Click here to read more about this), and that these lipids can influence the aggregation (or clustering) of alpha synuclein.
Remind me once again what is alpha synuclein?
In the Parkinsonian brain, a protein called alpha synuclein clumps (or aggregates) together, and this is believed to lead to the appearance of structures called Lewy bodies.
What are Lewy bodies?
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
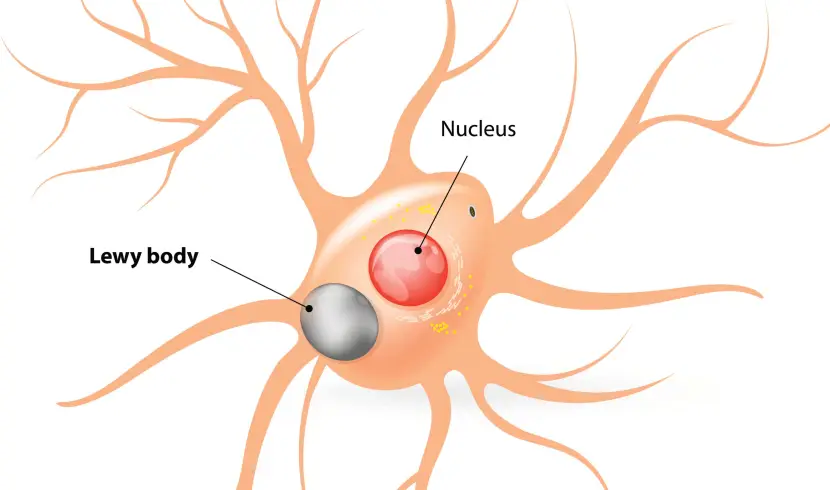 A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells. In the image below, alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (stained in brown; indicated by arrows). Source: Wikimedia
When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure. When it is first produced, alpha synuclein will look something like this:
 Alpha synuclein. Source: Wikipedia
Alpha synuclein. Source: Wikipedia
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of Monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein give rise to the aggregations of protein that go on to form the Lewy bodies we mentioned above. Certain versions of these two forms of alpha synuclein are also believed to possess toxic properties which in turn is considered to be influential in the progression of Parkinson’s.
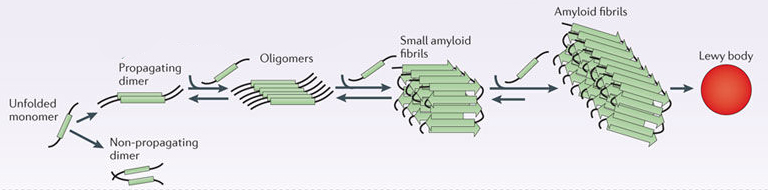 Parkinson’s associated alpha synuclein. Source: Nature
Parkinson’s associated alpha synuclein. Source: Nature
Have any particular lipids been found to influence alpha synuclein?
Yes.
Recently researchers in Boston (US) have published research highlighting a screening experiment in which they identified specific lipids that interact with alpha synuclein. Here is the report:

Title: Lipidomic Analysis of α-Synuclein Neurotoxicity Identifies Stearoyl CoA Desaturase as a Target for Parkinson Treatment
Authors: Fanning S, Haque A, Imberdis T, Baru V, Barrasa MI, Nuber S, Termine D, Ramalingam N, Ho GPH, Noble T, Sandoe J, Lou Y, Landgraf D, Freyzon Y, Newby G, Soldner F, Terry-Kantor E, Kim TE, Hofbauer HF, Becuwe M, Jaenisch R, Pincus D, Clish CB, Walther TC, Farese RV Jr, Srinivasan S, Welte MA, Kohlwein SD, Dettmer U, Lindquist S, Selkoe D.
Journal: Molecular Cell. 2018 Dec 1. pii: S1097-2765(18)30998-5.
PMID: 30527540
In this study, the investigators were interested in what happens with lipids in a situation of high levels of alpha synuclein. They decided to use yeast cells to explore this idea.
Why yeast cells?
Interesting question.
Quite possibly the earliest domesticated species, yeast is a single-celled microorganism, traditionally classified as a member of the fungus kingdom. The evolutionary lineage of yeast dates back hundreds of millions of years old and there are at least 1500 species of yeast (Source: Wikipedia).

The cellular structure of yeast. Source: Biocourseware
More importantly, yeast is one of the most centrally important model organisms used in modern biological research, representing one of the most thoroughly researched organisms in the world. And this is one of the reasons why the researchers used yeast in their screening experiment: We know more about the biology of yeast than we do about ourselves!
Why do scientists like studying yeast?
The main reason is that yeast cells are very similar to human cells, but they grow a lot faster (human cells on average divide a rate of about once every 12 hours, while yeast cells divide every two hours). This allows experiments to be conducted quicker. Yeast are also similar to human cells in that it has all of the eukaryote structures, including a nucleus, cytoplasm, and mitochondria (eukaryote meaning a cell with a nucleus). Thus, many of the discoveries made in yeast can be translated to human cells quite easily.
 Yeast cells. Source: NewEuropeans
Yeast cells. Source: NewEuropeans
More importantly, yeast was the first eukaryote (organisms whose cells have a nucleus) to have its DNA fully sequenced (in 1996). The genetic information collected from yeast DNA allowed molecular biologists to quickly move forward with their understanding of yeast biology, via genetic manipulation studies.
All of these advantages combined have meant that yeast are quite literally leading the way in biological research.
OK, so what did the researchers in this study do with their yeast cells?
Using yeast cells that produce very high levels of human alpha synuclein, the investigators found major changes in the lipidomics of these yeast cells (compared to normal control yeast cells). In particular, they observed large increases in diglycerides & triglycerides – two specific types of lipids.
Diglycerides are precursors to and triglycerides are key components of lipid droplets.
What are lipid droplets?
Lipid droplets, sometimes referred to as lipid bodies or adiposomes, are lipid-rich structures inside of cells.In the image below you can see lipid droplets (labelled ‘LD’) sitting beside mitochondria (labelled ‘M’).
 Source: Plosone
Source: Plosone
They are important structures inside almost every cell in the body. They are stores of energy for the cell, but they also respond to various changes in cellular conditions. Cellular stresses, in particular, such as starvation or pathogen invasions can evoke changes in lipid droplet metabolism and dynamics (Click here for a good OPEN ACCESS review of lipid droplets).
Did the researchers find an interesting connections between alpha synuclein and lipid droplets?
The researchers found that levels of alpha synuclein correlated with lipid droplet accumulation, which has been previously reported (Click here to read more about this).
Given this situation, the researchers wanted to test the effect of removing lipid droplets – whether it would have a protective or detrimental role – in the context of alpha synuclein toxicity. To assess the role of lipid droplets on alpha synuclein toxicity, the researchers engineered yeast cells lacking the two branches of enzymes required for lipid droplet production: genes involved in triglyceride production and genes involved in sterol ester production (and remember, a gene is a section of DNA that provides the instructions for making a specific protein/enzyme).
The removal of both pathways led to a decrease in the levels of lipid droplets, and an increase in alpha synuclein-related toxicity. This result suggested a protective role for lipid droplets production in the context of alpha synuclein toxicity.
When the researchers investigated the two branches individually, however, they noticed something interesting:
Removing just triglycerides increased alpha synuclein toxicity, while removal of sterol ester had no effect. This finding suggested to the investigators that triglycerides protect against alpha synuclein toxicity.
Next the researchers asked if high levels of alpha synuclein affects cellular lipid composition. Fatty acid profiling revealed that alpha synuclein expression caused a huge increase in levels of unsaturated fatty acids, particularly one called oleic acid.
 Oleic acid. Source: NIST
Oleic acid. Source: NIST
The investigators exposed alpha synuclein producing yeast cells to oleic acid and found that it robustly increased alpha synuclein toxicity. And by genetically removing enzymes involved in the production of oleic acid, they observed a significant reduction in alpha synuclein toxicity.
Next the researchers shifted their attention away from yeast cells and assessed whether these lipid effects relate to cells from other creatures. They firstly used rat cells grown in culture, human cells grown in culture, and also genetically engineered microscopic worms called C. Elegans. And they found the same results: by suppressing oleic acid overproduction, they could rescue cells from the toxic affects of alpha synuclein.
They also found that brain levels of unsaturated fatty acids, diglycerides, and triglycerides were significantly higher in mice with high levels of mutant human alpha synuclein (versus normal control mice).
The lab mouse. Source: Pinterest
Pharmacological inhibition of the oleic acid-generating enzyme ‘stearoyl-CoA-desaturase’ also dramatically decreased the levels of toxic alpha synuclein, without affecting the total amount of alpha synuclein. And the researchers suggested that this was in part due to the decreasing of the alpha synuclein tetramer:monomer ratio (Click here to read a previous SoPD post about this).
From all of these experiments, the researchers concluded that inhibition of monounsaturated fatty acid production – particularly oleic acid – can suppress alpha synuclein-induced degeneration. Thus, an inhibitor of the stearoyl-CoA-desaturase enzyme (which converts stearic acid to oleic acid) could represent an interesting therapeutic target for Parkinson’s.
Interesting. Wouldn’t it be great if there was an inhibitor of stearoyl-CoA-desaturase?
Yeah it would.
And guess what?
There just so happens to be a biotech company working on just such a compound.
Que?
At the same time as the above research report was published, this study report was also published:
 Title: Inhibiting Stearoyl-CoA Desaturase Ameliorates α-Synuclein Cytotoxicity
Title: Inhibiting Stearoyl-CoA Desaturase Ameliorates α-Synuclein Cytotoxicity
Authors: Vincent BM, Tardiff DF, Piotrowski JS, Aron R, Lucas MC, Chung CY, Bacherman H, Chen Y, Pires M, Subramaniam R, Doshi DB, Sadlish H, Raja WK, Solís EJ, Khurana V, Le Bourdonnec B, Scannevin RH, Rhodes KJ.
Journal: Cell Rep. 2018 Dec 4;25(10):2742-2754.e31.
PMID: 30517862 (This report is OPEN ACCESS if you would like to read it)
This research report was published by scientists – from a company called Yumanity – who identified a number of compounds that protected cells against alpha-synuclein-induced toxicity.
 Source: Yumanity
Source: Yumanity
Using yeast cells which produced high levels of alpha synuclein, the researchers who conducted this second study undertook a large screen for compounds that could protect the cells. They identified a series of strongly protective compounds, which contained a specific structure (a 1,2,4-oxadiazole core – don’t worry about what that means, just understand that it was a shared property across the protective compounds). And this rescue was specific to alpha synclein-associated toxicity as these compounds had no effect against toxic levels of Alzheimer’s-associated beta-amyloid protein or motor neurone disease-associated TDP-43 protein.
Curiously, all of these compounds had a specific dose range – as the dose increased, rescue was reduced and growth became inhibited. This observation suggested to the researchers that the 1,2,4-oxadiazole containing compounds were engaging a target involved in normal cell growth, but was also associated with alpha synuclein toxicity.
 Yeast. Source: Phys
Yeast. Source: Phys
The investigators used drug-resistant mutants screens to identify the targets of these compounds. This process involved causing spontaneous genetic mutations across yeast cells and then exposing those cells to one of the more potent compounds (called YTX-465). The cells that did not respond to YTX-465 were then isolated and their DNA was analysed. The researchers were looking for which genes (and remember, a gene is a section of DNA that provides the instructions for making a specific protein) were mutated, which would tell them which protein the compound was targeting.
When the investigators looks at the DNA from all of the compound-resistant cells, they found that all of the cells had a mutation in one particular gene.
Oooh, I wonder if you can guess which gene?…
…Drum roll please…
…the suspense is killing me…
…and the answer is: Oleic acid!
(For those of you who are reading all of this and not paying attention, thank you for your devotion, but Oleic acid is the very same target mentioned in the first research report discussed above).
The researchers found that adding oleic acid to yeast cell cultures reduced the effect of YTX-465 on alpha synuclein toxicity. They also found that high levels of YTX-465 inhibited the growth of normal yeast, suggesting that dosing may need to be carefully balanced in the inhibition of oleic acid.
Next the researchers wanted to shift from yeast cells to human cells to assess whether the inhibition of oleic acid could have beneficial properties in higher species. And as with the report above, they found that inhibition of the oleic acid producing enzyme ‘stearoyl-CoA desaturase’ did reduce the toxicity of alpha synuclein (even in neurons which carried the Parkinson’s-associated genetic mutation A53T).
Critically, the investigators were curious to determine if YTX-465 would work in human cells. They tested the compound along side another highly potent, commercially available stearoyl-CoA desaturase inhibitor called CAY10566 (also known as 28c). Their analysis revealed that YTX-465 inhibited oleic acid production in yeast, but not human cells, while CAY10566 was inactive against oleic acid production in yeast, but it was active in human cells. Thus, the investigators went back to the drawing board to design novel stearoyl-CoA desaturase inhibitors, which they are now testing.
Yumanity are seeking to begin clinical trials of some of these new inhibitor compounds in late 2019.
Excellent! Have there ever been any Stearoyl CoA desaturase inhibitors tested in the clinic?
Yes, there has been one.
The pharmaceutical company Merck developed a stearoyl-CoA desaturase inhibitor (called MK-8245) which was designed to target the liver:
 Title: Development of a liver-targeted stearoyl-CoA desaturase (SCD) inhibitor (MK-8245) to establish a therapeutic window for the treatment of diabetes and dyslipidemia.
Title: Development of a liver-targeted stearoyl-CoA desaturase (SCD) inhibitor (MK-8245) to establish a therapeutic window for the treatment of diabetes and dyslipidemia.
Authors: Oballa RM, Belair L, Black WC, Bleasby K, Chan CC, Desroches C, Du X, Gordon R, Guay J, Guiral S, Hafey MJ, Hamelin E, Huang Z, Kennedy B, Lachance N, Landry F, Li CS, Mancini J, Normandin D, Pocai A, Powell DA, Ramtohul YK, Skorey K, Sørensen D, Sturkenboom W, Styhler A, Waddleton DM, Wang H, Wong S, Xu L, Zhang L
Journal: J Med Chem. 2011 Jul 28; 54(14):5082-96.
PMID: 21661758
The researchers designed their drug to target the liver by using liver-specific organic anion transporting polypeptides. This mechanims was used to limit the exposure of other tissues to the effect of the stearoyl-CoA desaturase (SCD) inhibitor.
Merck conducted a Phase I clinical trial of MK-8245 in Type-2 diabetes mellitus in the USA (Click here to read more about this study), and the orally administered drug apparently produced no serious adverse events in any of the participants evaluated (Source).
The company also started a Phase IIa clinical trial, but that study was terminated (Click here to read more about this study)
Why did they stop? What went wrong?
The reason for stopping the clinical trial programme is unclear (“inability to recruit patients” is a cut-and-paste standard industry excuse).
Why haven’t other Stearoyl CoA desaturase inhibitors been developed for the clinic?
Not sure.
A lot of people are interested in the idea of inhibiting Stearoyl CoA desaturase, particularly for cancer (Click here for an example).
One of the big concerns about Stearoyl CoA desaturase inhibition, however, is that manipulation of the enzyme will result in different effects in different organs. Although inhibition of Stearoyl CoA desaturase may be good in some cells (such as alpha synuclein filled neurons), it could be very bad for other cells. For example, inhibition of Stearoyl CoA desaturase in models of colitis actually cause an increase in proinflammatory responses (Source) – and given the association between gut-associated issues and Parkinson’s, this could cause problems (Click here for a previous SoPD post on inflammatory bowel disease & PD). Thus, in a scenerio reminiscent of the early LRRK2 inhibitors, there could be some complications in non-CNS tissues that will need to be addressed.
Could Stearoyl CoA desaturase inhibitors be targetted to the brain?
Yes, the difference in effect across tissues was the reason why the Merck researchers designed MK-8245 to specifically target the liver, and some researchers are already suggesting that more brain-specific Stearoyl CoA desaturase enzymes could be targetted. There are different forms of Stearoyl CoA desaturase: Mice have 4 different forms of the enzyme (Scd1 through Scd4), while humans have just two: SCD1 and SCD5.
SCD5 is generally more confined to the brain. So perhaps, SCD5 is the better target.
But there could also be differences in Stearoyl CoA desaturase activity between different types of Parkinson’s. This kind of difference has been observed in preclinical models of other medical conditions. For example, a study that involved different preclinical models of diabetes found that Stearoyl CoA desaturase inhibition was beneficial in one genetic model, but detrimental in another (Source).
Thus, we will need to be rather cautious as we take a new class of drugs inhibiting Stearoyl CoA desaturase forward into clinical testing.
Ok, so we have to wait. But what about the Mediterranean diet we talked about at the top of this post?
Good point. And a very interesting point.
Oleic acid can be found in many different foods which are generally considered ‘healthy’. For example, avocadoes and olive oil.
But here’s the thing: Most of the fatty acids in our brain, are made by our brain. Fatty acids in our blood generally don’t enter the brain.
Despite this, some of the researchers behind the studies discussed today are looking into whether mice on low monounsaturated fats diet have more (or less) alpha synuclein issues in their brains. But this should not cause folks to pain and avoid the ‘benefits’ of the Mediterranean diet.
So what does it all mean?
There are some of these SoPD posts that are hard to write because in order to explain everything that is required to understand the topic, there is no way you can avoid the heavy biology lesson that often puts people to sleep. So if you are still reading this, I salute you. This post has been rather heavy.
The science is novel though, and (I hope you will agree) fascinating.
Two research groups from Boston have identified a whole new area of lipidomics that may be influencing Parkinson’s. In addition to opening new doors for the research community, this work is also rapidly developing a novel class of potential therapeutics for clinical testing next year.
It will be interesting to see how this research evolves, particularly considering the concerns mentioned about Stearoyl CoA desaturase inhibition across different tissues. But just as LRRK2 inhibitors have been re-engineered to avoid complications, hopefully the molecular biologists will be able to devise strategies that will allow for this new technology to be clinically tested and (if successful) ultimately used in the clinic.
More on this in 2019.
– – – – – – – – – – – – – – – – – –
One last note here:
Something that most readers will not be aware of is that one of the senior researchers on the research report discussed in this post was Prof Susan Lindquist.

Prof Susan Lindquist. Source: WallStreetJournal
She was truly a pioneer in the field of molecular biology. In the early 2000s, she suggested the idea of using yeast to look at neurodegenerative conditions like Parkinson’s. It was a wild concept. Talking to the New York Times in 2007, she explained that “Even people in my laboratory thought we were crazy to try to study neurodegenerative diseases with a yeast cell. It’s not a neuron”.
But she (and her lab) persevered and because of those efforts, we now not only have a new area of research for the Parkinson’s community to explore, but we also have a biotech firm (Yumanity) developing novel therapies based on that research. And we can hope that other biotech/pharma companies will follow.
Prof Lindquist sadly passed away in 2016, but such was her contribution to the field of Parkinson’s and neurodegenerative research that here she is still publishing as we approach 2019.
What an amazing legacy.
The banner for today’s post was sourced from bionumbers




Thanks Simon. I agree fully…fascinating, and a lot to digest.
LikeLike
Thanks Tom – glad you liked it.
Simon
LikeLike
In one post, you’ve linked together the short-chain fatty acid gut-brain connection observations of Mazmanian’s group at Caltech, the tetramer/monomer ratio of your recent post, and, perhaps coincidentally, the link to diabetes medication, along with really interesting new research targets. Makes me wish I’d studied bio-chemistry! It also ‘feels’ like we’re getting really close to an understanding of a large fraction of the various disease mechanisms involved in Parkinson’s. Thank you again for your tireless efforts to explain and clarify some of this fascinating research for us. It gives me great hope ( as well as keeping me entertained).
LikeLike
You are welcome Tom! If nothing else, I am here to entertain. And I hope you are right that we are getting close to figuring all of this out. Here’s to getting it done in 2019!
Kind regards,
Simon
LikeLike
Dear Simon;
I can not open the “Science of Parkinson’s” link since couple of days. Is there anything wrong with the address? Is there an alternative address through which I can reach your site.
Regards
Tayfur Cinemre
LikeLike
Hi Tayfur,
Sorry to hear this. I am not sure why you would be having trouble accessing the website. Everything is functioning just fine as far as I am aware. Is there a particular link you are referring to?
Kind regards,
Simon
LikeLike