
|
The clustering of a protein called alpha synuclein is one of the cardinal features of the brain of a person with Parkinson’s disease. Recently published research has demonstrated that tiny antibodies (called nanobodies) derived from llamas (yes, llamas) are very effective at reducing this clustering of alpha synuclein in cell culture models of Parkinson’s disease. In today’s post, we will discuss the science, review the research and consider what it could all mean for Parkinson’s disease. |

Llama. Source: Imagesanimals
Ok, I confess: This post has been partly written purely because I really like llamas. And I’m not ashamed to admit it either.
I mean, look at them! They are fantastic:

Source: Vogue
Very cute. But what does this have to do with Parkinson’s disease?
Indeed. Let’s get down to business.
This post has also been written because llamas have a very interesting biological characteristic that is now being exploited in many areas of medical research, including for Parkinson’s disease.
Last week this research report was published:

Title: Nanobodies raised against monomeric ɑ-synuclein inhibit fibril formation and destabilize toxic oligomeric species
Authors: Iljina M, Hong L, Horrocks MH, Ludtmann MH, Choi ML, Hughes CD, Ruggeri FS, Guilliams T, Buell AK, Lee JE, Gandhi S, Lee SF, Bryant CE, Vendruscolo M, Knowles TPJ, Dobson CM, De Genst E, Klenerman D.
Journal: BMC Biology. 2017 Jul 3;15(1):57.
PMID: 28673288 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers used a special kind of antibody that was made in llamas to target the toxic form of the protein alpha synuclein – perceived public enemy #1 in the world of Parkinson’s disease. The clustering of alpha synuclein protein is believed to be responsible for the cell loss that is associated with the condition.
Why Llamas?
Great question. Fascinating answer.
So Llamas are members of the Camelidae family (which include alpacas, dromedaries and camels).

Meet the Camelidaes. Source: Pinterest
A very interesting feature of the Camelidae family is that in addition to having normal antibodies, they also have another class of naturally occurring antibodies that are devoid of light chains, which researchers have further adapted into mini antibodies that we call a “nanobody”.
Hang on a second. Slow down. What are antibodies, light chains and nanobodies?
An antibody is a critical part of our immune system.
When a pathogen (an agent that causes disease or damage) is detected in your body, it will quickly be determined to be not ‘self’ (meaning that it is not part of ‘you’ yourself). This judgement will be made by the identification of antigens on the surface of the pathogen. An antigen is defined as any substance or molecule that is capable of causing an immune response in an organism. If an molecule on the surface of the pathogen is not familiar to the immune system, it will be considered an antigen and an immune response will be initiated.
A good example of a pathogen is the common cold virus. Once inside the body, the presence of the virus will be detected by cells in the immune system and given that the virus will be presenting antigens on its surface that are clearly not self, an immune response will be initiated. The cells that carry out the immune response are white blood cells known as lymphocytes.

That big cell in the middle is a lymphocyte. Source: ASH
There are basically two types of immune response:
- An antibody response
- A cell-mediated immune response
These processes are carried out by two different types of lymphocyte cells (B cells and T cells). In the antibody response, B cells are activated and they begin to secrete Y-shaped proteins called antibodies. These are used by the immune system to label and neutralise foreign or dangerous substances.

Antibodies binding to a virus. Source: Biology-questions-and-answers
Antibodies bind to parts of the antigen called epitopes. Also known as antigenic determinants, an epitope is the part of an antigen that is recognised by an antibody. Antibodies by themselves can do a pretty good job of stopping pathogens, by blocking them from attaching to cells or by sticking together and clustering the antigens to prevent them from doing anything bad.

Antibody binding to antigens. Source: Venngage
Ok, but what is a light chain?
The “immunoglobulin light chain” (by its full name) is a component of an antibody.
Your typical antibody is composed of two immunoglobulin heavy chains and two immunoglobulin light chains:

The light chains are in pink. Source: Bitesizebio
Most of the structure of an antibody is constant (always the same), but the top of the Y arms are always variable (or changing from antibody to antibody). These variable parts are the antigen identifying and binding regions, while the bottom stalk of the Y is the part the rest of the immune system interacts with.
Ok, so camels and llamas have antibodies without these light chains?
Members of the Camelidae family (alpacas, dromedaries and camels) have both normal antibodies and these other antibodies without these light chains (we will call them ‘heavy chain only antibodies’). And this second kind of antibody is rather unique in the animal kingdom. Only sharks and the Camelidae family have them.

Source: ABCnews
These heavy chain only antibodies have similar potency, specificity and diversity as the conventional antibodies and they have evolved to play an active part in the immune system of these animals.
So what are nanobodies?
Nanobodies are a small fragment of the heavy chain only antibodies, consisting of just a single arm of the heavy chain (also called a monomeric variable antibody domain).

The difference between antibodies and nanobodies. Source: SteyaertLab
Due to their tiny size and unique structure, nanobodies offer some very important advantages over conventional antibodies – especially when considering their use in therapeutics:
- Nanobodies can be combined with each other (up to 7 in one go), or be combined with other molecules or drugs, allowing for multiple targets to be addressed in one combined drug.
- Nanobodies can bind with epitopes on targets which are hidden or shielded from the much larger conventional antibodies.
- They are significantly more stable and aggregation resistant compared to conventional antibodies.
- Nanobodies have a larger spectrum of antigenic epitopes, including enzyme active sites.
While a lot of the current medications in the clinic (or going through clinical trial) are based on conventional antibody technology, the future will probably be focused on nanobodies. In fact, there is a Belgian biotech company called Ablynx that currently has more than 45 nanobody-based therapeutic research programs.

Eight of their nanobodies are in various stages of clinical trial testing, and their first potential product (Caplacizumab for the treatment of acquired Thrombotic thrombocytopenic purpura – is a rare blood disorder) is expected to be approved for clinical use in 2018. At present they do not have any clinical programs for brain related conditions (like Alzheimer’s and Parkinson’s).
But this could potentially change as a result of the research published last week:

Title: Nanobodies raised against monomeric ɑ-synuclein inhibit fibril formation and destabilize toxic oligomeric species
Authors: Iljina M, Hong L, Horrocks MH, Ludtmann MH, Choi ML, Hughes CD, Ruggeri FS, Guilliams T, Buell AK, Lee JE, Gandhi S, Lee SF, Bryant CE, Vendruscolo M, Knowles TPJ, Dobson CM, De Genst E, Klenerman D.
Journal: BMC Biology. 2017 Jul 3;15(1):57.
PMID: 28673288 (This article is OPEN ACCESS if you would like to read it)
The researchers conducting this study had previously identified two nanobodies, called NbSyn2 and NbSyn87, which bind to particular epitopes on the protein alpha synuclein (on residues 137–140 and 118–131, respectively). The nanobodies were created by immunising (or injecting) llamas with a section of human alpha synuclein protein.
Why did they use llamas though?
Let me answer this by asking you a question: if you had the choice between injecting stuff into man-eating sharks, spit-in-your-face camels, or cute & cuddly llamas, which would you choose?
Seriously though, there is a good reason why we use llamas rather than man-eating sharks: although sharks have heavy-chain only antibodies (better known as new antigen receptors or NARs), they are actually very different from mammalian heavy chain antibodies.

Shark NARs (left) are different to Camelid hclgG & human IgG. Source: Wikipedia
In evolutionary terms, sharks separated from mammals a very long time ago, and this shows itself not only in the different shape of the NARs (see image above), but also in the very high similarity between Camelid hclgG & human IgG. An important consequence of this fact is that camelid Ig is expected to be much less immunogenic in human than the NARs.
The antibodies retrieved from the llamas were then researched and chemically altered giving rise to the two nanobodies that the investigators conducted experiments on in this current research report – Click here and here to read the research behind the discovery of those NbSyn2 and NbSyn87, respectively.
Why were they targeting alpha synuclein?
Alpha synuclein is a protein that is believed to be responsible for the spread of Parkinson’s disease in the brain as well as the cell death that is associated with the condition. The loss of cells is generally believed to be caused by a toxic form of alpha synuclein which clusters (or aggregates) together and forms Lewy bodies.
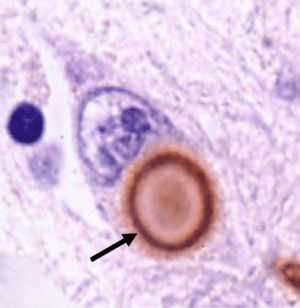
What are Lewy bodies?
Lewy bodies are circular clumps of alpha synuclein (and other proteins) that are found in the brains of people with Parkinson’s disease. They are one of the characteristic features of the Parkinsonian brain, and they are very abundant in areas of the brain that have suffered cell loss, such as the region containing dopamine neurons.
A lewy body (brown with a black arrow) inside a cell. Source: Cure Dementia
How do Lewy bodies form?
We don’t really know.
No one has ever seen the process of lewy body formation and all we can do is speculate. Currently there is a lot of evidence supporting the idea that the toxic version of alpha synuclein can be passed between cells. Once inside the new cell, the toxic alpha synuclein helps to seed the formation of new Lewy bodies, and this is how the disease is believed to progress.
The passing of alpha synuclein between brain cells. Source: Nature
Thus, there is a lot of research effort going into finding new therapeutics that a.) stop the spread of alpha synuclein in the brain, and b.) stop alpha synuclein from clustering and forming the toxic version (also called oligomers) of this protein.
And what do the llama nanobodies do?
When they tested the two nanobodies, the investigators found that both NbSyn2 and NbSyn87 inhibit the formation of the toxic form of alpha synuclein. NbSyn87, in particular was very effective at preventing individual alpha synuclein proteins from binding together and forming the stick-like structures that ultimately go on to aggregate further and (presumably) ultimately form Lewy bodies.
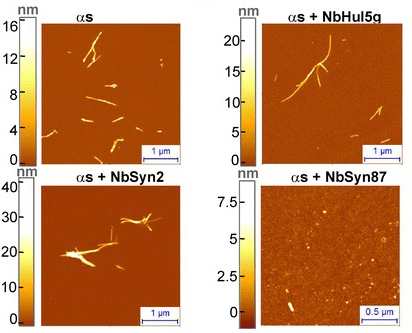
Source: Ncbi
Furthermore, the researchers demonstrated that nanobody binding promoted a rapid structural conversion of already aggregated alpha synuclein into the less clustered version of the protein. Importantly, this conversion lead to a dramatic reduction in alpha synuclein-induced cellular toxicity in cells grown in culture. The researchers are now following up this research by looking at whether this de-aggregating effect can work in animal models of Parkinson’s disease.
I’ll be looking out for that research when it is published.
What does it all mean?
It means that a unique biological property of llamas is helping researchers not only to better understand Parkinson’s disease, but also potentially providing us with a novel future therapy for the condition. The mini antibodies (nanobodies) are demonstrating efficacy in many other areas of medical research – both clinically and pre-clinically – perhaps it is time they received more attention in the Parkinson’s research world.
If current antibody (immunotherapy) clinical trials demonstrate that they can effectively slow down/halt Parkinson’s disease, then nanobodies targeting alpha synuclein should definitely warrant further investigation (Click here to read more about the immunotherapy clinical trials).
 The author of this blog would like to thank Dr. Erwin De Genst & Dr. Marija Iljina (the researchers behind the nanobody study reviewed in this post) for their immediate and generous help when I approached them about writing this post. It was greatly appreciated.
The author of this blog would like to thank Dr. Erwin De Genst & Dr. Marija Iljina (the researchers behind the nanobody study reviewed in this post) for their immediate and generous help when I approached them about writing this post. It was greatly appreciated.
For more information about this study, I recommend reading their write up on the Biomedcentral blog.

One last llama image. Source: Independent
The banner for today’s post was sourced from Pinterest

An excellent and witty article..line up the fruit flies! 🙂
LikeLike
Hi Lisa,
Thanks for the comment and glad you liked the post. Adorable woolly creatures make for interesting content.
Kind regards,
Simon
LikeLiked by 1 person
Cool, cuddly and maybe a cure!
LikeLike
Indeed! Glad you liked it.
LikeLike
It turns out there are a number of over the counter supplements that inhibit alpha-synuclein aggregation, and some can even disaggregate it. So while llamas are bizarrely cute (my nephew shears them professionally), they are not giving up their disaggregating secrets the way that curcumin, baicalin, EGCg and quercetin already have.
This article provides a good list of mostly over the counter AS disaggregators:
https://www.frontiersin.org/article/10.3389/fphar.2018.01555/full
LikeLike