|
In a recent SoPD post, we discussed the importance of calcium and looked at how it interacts with the Parkinson’s-associated protein alpha synuclein, affecting the function and clustering of that protein. During the writing of that post, another interesting research report was published on the same topic of calcium and alpha synuclein. It involved a different aspect of biology in the cell – a structure called the endoplasmic reticulum – but the findings of that study could also explain some aspects of Parkinson’s. In today’s post, we will review the new research report, consider the biology behind the findings and how it could relate to Parkinson’s, and discuss how this new information could be used. |
The original berserker. Source: Wikipedia
I can remember my father often saying “If you kids don’t be quiet, I’ll go berserk!”
Growing up, I never questioned the meaning of the word ‘berserk‘.
I simply took it as defining the state of mindless madness that my dad could potentially enter if we – his off-spring – pushed him a wee bit too far (and for the record, Dad actually ‘going berserk’ was a very rare event).
My father. But only on the odd occasion. Source: Screenrant
But now as I find myself repeating these same words to my own off-spring, I am left wondering what on Earth it actually means?
What is ‘berserk‘?
According to Wikipedia, the word stems from ‘Berserker‘ which was the name given to “champion Norse warriors who are primarily reported in Icelandic sagas to have fought in a trance-like fury”. In battle, the berserkers would be overcome by fits of rage, become immune to pain, and wreak havoc amidst enemy lines.
The English word ‘berserk’ is believed to be derived from the Old Norse words ‘ber-serkr’ (plural ber-serkir) which could mean “bear-shirt”, and some have suggested that this may result from warriors wearing bear skins over themselves as they charged at the front lines.
Spot the bear skin-clad berserker. Source: Wikipedia
The bear skin may have also served as a method of identifying individuals who in the midst of the trance-like battle fury – which was called berserkergang – should be avoided on the battle field as they would not be able to tell friend from foe.
To be fair, my father never quite reached that level of berserk-ness, and I hope I don’t ever either (though I am sorely tested sometimes).
What does this have to do with Parkinson’s?
Well, some research has recently been published that suggests that the clustered (or aggregated) form of the Parkinson’s-associated protein alpha synuclein could be causing certain biological processes inside the cell to go…well, ‘berserk’:
Title: Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation.
Authors: Betzer C, Lassen LB, Olsen A, Kofoed RH, Reimer L, Gregersen E, Zheng J, Calì T, Gai WP, Chen T, Moeller A, Brini M, Fu Y, Halliday G, Brudek T, Aznar S, Pakkenberg B, Andersen JP, Jensen PH.
Journal: EMBO Rep. 2018 Mar 29.
PMID: 29599149 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to investigate the relationship between calcium and the aggregation of alpha synuclein. But rather than focusing on the vesicles in the synapse (like the report reviewed in our previous post – click here to read that), these scientists focused on what was happening in the main body of the cell.
They started their investigation by looking at what happens to calcium levels inside cells when the amount of alpha synuclein being produced in the cells goes up. Compared to cells with normal levels of alpha synuclein, the investigators found that calcium levels firstly drop and before later increasing in cells with very high levels of alpha synuclein. The time frame differed between different types of cells (and cell lines), but the pattern/trend was the same:
Source: EMBO
In the graph above, cytosolic calcium refers to the calcium floating around inside the cell, but not inside specific structures in the cell (such as the nucleus). The fluid inside the cell is referred to as the cytosol.
Next, the researchers wanted to look inside the cell and see how alpha synuclein could be causing this curious pattern in cytosolic calcium levels. Given that previous research has suggested that aggregated alpha synuclein often localises to the endoplasmic reticulum (Click here for that research), the investigators turned their attention to that structure first.
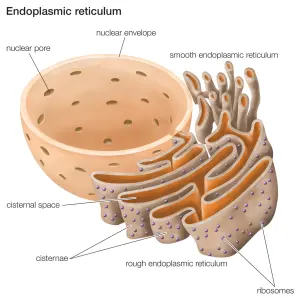
What is the endoplasmic reticulum?
The endoplasmic reticulum (or ER) is the assembly line where proteins are produced in a cell. It is closely attached to the nucleus of the cell.
The endoplasmic reticulum. Source: Britannica
The nucleus is where the blue prints/designs for each protein are kept in the form of DNA. A template of each plan for a protein can be generated (in the form of RNA), and that is converted (via a process called translation) into protein. A large part of that protein production process is conducted in the endoplasmic reticulum.
This video explains exactly what the ER does:
Now there is a big difference between the levels of calcium in the endoplasmic reticulum and the cytosol (approximately 0.5 mM inside the ER, compared to a concentration of 100 nM in cytosol). This rather steep gradient is maintained by a protein that sits in the wall of the endoplasmic reticulum.
That protein is called ‘sarco/endoplasmic reticulum Ca2+-ATPase’ (or simply SERCA).
SERCA. Source: slideplayer
SERCA is a pump that transfers calcium from the cytosol of the cell into the space inside the endoplasmic reticulum (that space is called the lumen). The SERCA represented one method by which the cell could lower levels of calcium in the cytosol. But this would require SERCA to actively remove calcium from the cytosol against the high concentrations of calcium inside the endoplasmic reticulum.
Thus, the question facing the researchers was how would this work?
What the researchers found was rather startling: the aggregated alpha synuclein actually bound itself to and activated SERCA.
And this resulted sustained activity of the SERCA pump which caused the lowering of levels of cytosolic calcium, and higher levels of calcium in the endoplasmic reticulum. Inhibitors of alpha synuclein aggregation could block this effect, and aggregation-inducing proteins could exaggerate it. As you can see in the graph below calcium (Ca2+) transport into the endoplasmic reticulum increases in the presence of alpha synuclein (AS), and increases further in the presence of alpha synuclein and p25a (an experimental aggregation‐inducing protein).
Source: EMBO
The investigators found that a SERCA inhibitor (called cyclopiazonic acid) could normalise both the initial reduction seen in cells producing high levels of alpha synuclein and the later increase in cytosolic calcium.
The activation of SERCA by aggregated alpha synuclein also resulted in 30-40% cell loss after approximately 14 days in the culture. And the researchers were confident that this over-active SERCA phenomenon was involved in the cell death, because when they treated the cells with cyclopiazonic acid – the SERCA inhibitor – the cells survived. As you can see in the graph below, normal cells (WT) treated with a ‘vehicle’ control solution or cyclopiazonic acid (CPA) presented no difference in loss of neurons. The cells producing too much aggregating alpha synuclein (AS), however, suffered cell loss unless they were treated with CPA:
Source: EMBO
The increase in loss of cells was associated with the late increase in cytosolic calcium levels that we observed in the figure above (reproduced below). As the cells began to die, the levels of cytosolic calcium rose well above average. And this effect was rescued by treating the cells with the SERCA inhibitor, cyclopiazonic acid.
Source: EMBO
The researchers next treated some microscopic worms (called Caenorhabditis elegans) that had been genetically engineered to produce high levels of alpha synuclein, with CPA and they found that this treatment protected the dopaminergic neurons in these organisms against the alpha synuclein‐associated degeneration.
And finally, the investigators wanted to see if these findings could be relevant to the human situation, so they conducted an analysis of human postmortem brains and demonstrated that aggregated alpha synuclein was very closely proximity to SERCA in people with dementia with Lewy bodies (DLB – a condition similar to Parkinson’s) but not in healthy control (Ctrl) samples. They used a technique that only labels the tissue (red) if the two proteins are both in the same place, and they found that only the sections of brain from people with DLB had any red staining (which was always close to the nucleus (blue in the image below):
Alpha synclein and SERCA (co-labelled in red) in DLB samples. Source: EMBO
The researchers concluded that their results suggest that aggregates of alpha synuclein bind to SERCA and stimulate its activity. Reducing SERCA activity is neuroprotective, and they suggested that SERCA and associated processes may be novel therapeutic targets for treating Parkinson’s.
Are there any clinically available SERCA inhibitors?
So there is a SERCA inhibitor that is currently in clinical trials for brain cancer.
It is a derivative of thapsigargin, called mipsagargin (also named G-202). A biotech called Inspyr Therapeutics (no website; previously called ‘GenSpera’) is currently undergoing clinical trials for the treatment of glioblastoma (Click here to read more about this trial).
Inspyr Therapeutics
While this intravenously injected drug being tested in cancer, it could be interesting to investigate its use in models of Parkinson’s.
But rather than targeting SERCA with inhibitors, there may be a better approach to reducing the berserk activity of this pump that is currently being clinically tested for Parkinson’s.
Which is?
Well, if aggregated alpha synuclein is binding to SERCA and causing it to… go berserk, then would it not make sense to try and reduce the amount of aggregated alpha synuclein?
And we are already clinically testing treatments that do just that in Parkinson’s.
One way of dealing with the aggregated alpha synuclein is to grab it as it is being passed between cells (and then remove it from the body). The passing of aggregated alpha synuclein between cells is one possible method by which Parkinson’s progresses over time.
And this idea has given rise to a series of ongoing clinical trials that are using a process called immunotherapy – which involves boosting the body’s immune system to target toxic forms of alpha synuclein.

Monoclonal antibodies. Source: Astrazeneca
This immunotherapy approach uses antibodies, which are Y-shaped proteins that act like alert flags for the immune system. Antibodies target very specific structures, while ignoring everything else. Immunotherapy can be conducted in two way
- The body’s immune system can be encouraged to target the toxic form of alpha synuclein (using active immunisation in the form of a vaccine); or
- Researchers can design antibodies themselves that specifically target the toxic form of alpha synuclein (while leaving the normal version of the protein alone), and then inject those antibodies into the body (passive immunisation)
Immunotherapy. Source: Acimmune
Both of these approaches are being tested in two clinical trial programmes are being run by two biotech companies.
The vaccine approach is being tested by an Austrian biotech firm called AFFiRiS.
In June 2017, AFFiRiS announced that the results of Phase I clinical study of their product AFFITOPE® PD03A. The company reported that the vaccine is causing an immune response (the immune system is generating antibodies against the toxic form of alpha synuclein) and that the vaccine was safe in people with Parkinson’s (Click here to read the press release, and click here to read more about the trial).
AFFiRiS has two vaccines for Parkinson’s that have been studied in phase I studies, and thus far 98 people with Parkinson’s or multiple system atrophy (MSA, a condition very similar to Parkinson’s – Click here to read a SoPD post on this topic) have participated in studies investigating either AFFITOPE® PD01A or PD03A. During these studies, participants were observed for up to 48 months (AFFITOPE® PD01A) or 12 months (AFFITOPE® PD03A), respectively. These observations have focused on long-term safety, immunological and clinical parameters, and the vaccine appears to be relatively safe.
Next, the company will be seeking to determine if they actually work. AFFiRiS is currently planning a Phase II efficacy trial, which we should hear more about shortly. (It would also be nice to hear about the follow up analysis of the 90+ individuals who have received the vaccine – have they demonstrated any benefits?).
Vaccination. Source: WebMD
Meanwhile, the passive immunisation (or ‘design your own antibodies’) approach to immunotherapy is being tested by an American biotech company called Prothena.
In 2016, Prothena reported the Phase I safety clinical trial results of their primary drug PRX002 (also known as RG7935; Click here to read the research report on this study), and they have rapidly moved on to Phase II testing of the drug, which is called the PASADENA study (listed on the Clinicaltrials.gov as NCT03100149).
That study involves two parts. Part 1 is a randomised, double-blind, placebo-controlled, three-arm study which will enrol approximately 300 patients (less than 2 years since diagnosis) to evaluate the efficacy and safety of PRX002 in people with Parkinson’s over 52 weeks. Participants will be randomly assigned to one of three groups (1500 mg or 4500 mg of PRX002, or placebo treatment). The treatments will be administered via intravenous infusion once every 4 weeks. One complicating aspect of this first part of the study is that eligible participants must not be on any dopaminergic therapy, and they must not be expected to require dopaminergic therapy for the 52 weeks of Part 1 of the study – identifying subjects that fit this criteria may slow recruitment.
Part 2 of this Phase II clinical trial is a 52-week blinded extension phase in which participants from the placebo group of the study will be re-randomly assigned into one of two active doses on a 1:1 basis. This means that all participants will be on active treatment. Participants who were originally assigned to an active dose will continue at that same dose level for the additional 52 weeks. In part 2 of the study, participants will be allowed to use dopaminergic therapies (if required).
We are not expecting the results of this trial until 2020/21.
And AFFiRiS and Prothena are not the only companies investigating the immunotherapy approach for Parkinson’s. There are other companies with early-stage programs in this area, including Biogen (BIIB054), AC Immune (ACI-870), Proclara (NPT088),NeuroPore (NPT200-11; in collaboration with UCB) and BioArctic Neuroscience (BAN0805; in collaboration with AbbVie).
If antibodies can reduce the levels of aggregated alpha synuclein in the brain, perhaps this would reduce the ‘berserk’ over activation of SERCA. It will be interesting to see.
|
A topic for discussion: Recently, we have seen evidence of anti-inflammatory benefits in neurodegeneration, a key role for calcium in models of PD, and now further evidence of alpha synuclein involvement (today’s post). Could it be that we are heading for a future where multiple treatments will be required for dealing with PD? For example, a treatment to reduce the detrimental effects of aggregated alpha synuclein, a calcium blocker to reduce the stress being placed on the cells, AND an anti-inflammatory agent to quell the immune system response. Are all three of these treatment components required? And is it a mistake to clinically test each of these treatment components in isolation? |
What does it all mean?
Researchers in Denmark have discovered another biological pathway that is affected by the bad boy of Parkinson’s, alpha synuclein. The aggregated form of this protein has been found to bind to and over-activate a calcium pump inside of cells which results in stress being caused to specific components of the cell and ultimately results in cell death. The investigators also identified a method (inhibition of the pump) that not only returned calcium levels to normal, but also protected the cells from dying.
While the results are very interesting, like all good studies this one has raised many new questions that need to be answered. For example, if this effect occurs in most cells affected by alpha synuclein, why do only certain cells (such as the dopamine-producing neurons) particularly vulnerable to neurodegeneration in Parkinson’s.
It will be necessary to firstly have the results of the current study independently replicated (there are probably research groups already doing this) and if found to be reliably valid, then work can start on screening for selective inhibitors of the pump to add further treatment options to our growing arsenal of drugs for Parkinson’s (and there are probably research groups already doing this).
And if those inhibitors work for PD, then maybe they can be repurposed for fathers at risk of going berserk (I think there’s a massive market opportunity for medication that helps parents dealing with ‘three-nagers’).
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, many of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from slideplayer
















Hello. I find your posts very readable and useful (thank you very much). Regarding the 2 posts in May and aggregation of Alpha-Syn in general is there any relationship to what role mannitol (pre-clinically) may/may not play in all this interaction (re: 2 posts in 2016). Btw not using mannitol myself. Thx.
LikeLike
Hi John,
Thanks for your comment – glad you like the material.
For the readers who are not familiar with Mannitol, it is an Food and Drug Administration (FDA)-approved compound to be used as an osmotic diuretic agent. It is widely used as a sweetener. In 2013, Israeli researchers demonstrated that low doses (but not high doses) were rather effective at reducing the aggregation of alpha synuclein (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3682557/ or https://scienceofparkinsons.com/2016/05/10/manna-from-heaven-mannitol-and-parkinsons-disease/). Subsequent to this, a group in Israel set up an online community called ‘Clinicrowd’ (https://clinicrowd.info/) with the purpose of trying to determine if there are benefits to using mannitol for the treatment of Parkinson’s (for more: https://scienceofparkinsons.com/2016/12/28/update-mannitol-and-parkinsons-disease/).
I have recently been in contact with the good folks at Clinicrowd and they have something (results-wise) in the works with regards to mannitol. I’m not sure about the timeframe of it being published, but they will hopefully have some news coming soon. When that report is available, we’ll have a look at it here on the SoPD.
The unfortunate part of the mannitol story is the lack of follow up work since the original research (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3682557/) was published in 2013. I understand that Prof Segal and his team in Israel are still working on it, but the rest of the research community hasn’t jumped on the band wagon yet. This leaves a bit of a vacuum with regards to new information. And the absence of independent replication of the research makes it very hard to form any kind of opinion on the matter – and this is an issue with many areas of PD research, I’m not singling out mannitol here.
One of the key issues with using Mannitol as a ‘treatment’ is that it is poorly absorbed from the gut (see this excellent open-access review for a good background on this: http://ceaccp.oxfordjournals.org/content/early/2012/01/12/bjaceaccp.mkr063.full). So it will be very interesting to see what results Clinicrowd have regarding Mannitol and Parkinson’s.
I hope this helps.
Kind regards,
Simon
LikeLike
Hi Simon,
Do we know if Ca channel blockers that are routinely used for treatment of high blood pressure have any effect on neurons? If so how do you think they affect SERCA berserker system? I found these quite interesting studies:
https://www.ncbi.nlm.nih.gov/pubmed/25845582
https://www.ncbi.nlm.nih.gov/pubmed/18591113
Thank you,
Felix
LikeLike
Hi Felix,
Thanks for your comment and interesting question. In a previous post we dealt with the topic of Ca Channel blockers (CCBs) an Parkinson’s (https://scienceofparkinsons.com/2018/05/01/calcium/), but it is important to understand that not all CCBs are the same. There are different types (or classes) of CCBs. There is actually one class of CCBs that have been associated with the appearance of Parkinsonisms (Parkinson’s-like features, such as tremor – https://www.ncbi.nlm.nih.gov/pubmed/18591113). That class of CCBs is called diphenylmethylpiperazines (Cinnarizine & flunarizine are common versions of this – https://www.ncbi.nlm.nih.gov/pubmed/15120099).
The CCBs that are currently believed to be beneficial are the dihydropyridine class CCBs (such as Isradipine which is being clinically tested in the STEADY-PD study – https://steadypd3.com/). The results of the trial should be available later next year (2019). I am not sure that CCBs would have much impact on SERCA. CCBs have most of their effect on the cell surface and I don’t think they get inside the cell and do anything. If this ‘SERCA going berserker’ mechanism is independently replicated, the best approach to stopping aggregated alpha synuclein from activating SERCA will be treatments that either block the spread of toxic alpha synuclein (as we discussed in this post) OR treatments that boosts the waste disposal system of the cell, helping the cell to remove excess alpha synuclein (such as Nilotinib and Ambroxol which are currently being clinically tested; we discuss these in the outlook for 2018 post – https://scienceofparkinsons.com/2018/01/07/2018/).
I hope this answers your question. Let me know if anything isn’t clear.
Kind regards,
Simon
LikeLike
Simon you have done it again! I was quite confident that my understanding of the myriad functions of calcium was clear enough to inform my nutrient needs accurately enough. Now? Hey who knows to within a 20 % degree of accuracy? As is ever the case, each new answer drags its bedraggled offspring and step-children up to the table where they indulge themselves in the minds of Parky researchers, often bringing forth many more questions (and their multiplication of answers etc, etc, etc.). The potential for obfuscation is off the charts!
The ironic thing is this is THE ESSENCE of Parky research – the more questions the greater the chance that we (you and many others) will eventually answer the questions that needed to be asked (Oh woe, why did we not know to ask and answer these questions which have unlocked the Arabian nights cave – it was a bit harder than just saying ‘open sesame!)’.
And that’s why I say to everyone ‘Have you heard of SIMON STOTT?’ If not (i know I am preaching to the converted) it is up to us to spread the word and get more people reading his superb blog and learning a broader understanding of the SCIENCE OF PARKINSONS!
That’s all folks from me this time
Lionelljp
LikeLike
Hi Lionel,
Thanks for screaming it from the roof tops for me – much appreciated. Let me know some of those questions some time.
Kind regards,
Simon
LikeLike