|
Graphene is widely being believed to be one of the building blocks of the future. This revolutionary 2D material is being considered for all kinds of applications, including those of a medicinal nature. This week researchers from the John Hopkins University School of Medicine and Seoul National University have published a report suggesting that graphene may also have applications for Parkinson’s. The researchers found that exposing the Parkinson’s-associated protein, alpha synuclein, to graphene quantum dots not only prevented the protein from aggregating together into its toxic form, but also destroyed the mature toxic form of it. A nano-sized silver bullet? In today’s post, we will look at what graphene quantum dots are, review the new Parkinson’s-related results, and discuss what happens next for this new technology. |
Prof Andre Geim and Prof Konstantin Novoselov. Source: Aerogelgraphene
They called them ‘Friday night experiments’.
Each week, two research scientists at the University of Manchester (UK) named Andre Geim and Konstantin Novoselov held sessions where they would conduct experiments that had little or nothing to do with their actual research.
These activities were simply an exercise in genuine curiosity.
And on one particular Friday in 2004, the two scientists conducted one of the simplest experiments that they had ever attempted – but it was one which would change the world: They took some sticky tape and applied it to a lump of graphite.
What is graphite?
The dark, hard stuff on the right: That’s graphite. Source: Wikipedia
Graphite is one of the three naturally occurring physical forms of the element carbon (the three forms being diamond, graphite, and charcoal (or amorphous carbon) – a, b & c respectively in the image below).
Diamond (a), graphite (b) and coal (c). Source: Wikipedia
Looking at the image above, you will notice that graphite (b) is made up of 2 dimensional layer upon 2 dimension layer of carbon sheets.
These 2 dimensional sheets of carbon are called graphene.
And since the 1950s, physicists had been trying to generate single layers of graphene. But before 2004, those attempts never got less than 50 to 100 layers thick.
Now back to Geim and Novoselov: Using their sticky tape, they removed some flakes of graphite.
Flakes of graphite on sticky tape. Source: Modisulfide
As they did this, they noticed some flakes were thinner than others, and this got them wondering ‘how thin can we go?’. Genuine curiosity right? By separating the graphite fragments repeatedly (using lots of sticky tape), they managed to create flakes that were just one atom thick in places.
It was the first time graphene had ever been isolated.
They published a report about their finding in 2004:
Title: Electric field effect in atomically thin carbon films
Authors: Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA.
Journal: Science, 2004 Oct 22;306(5696):666-9.
PMID: 15499015
And in 2010, the Nobel Committee for Physics at the Royal Swedish Academy of Sciences awarded the 2010 Nobel Prize in Physics to Andre Geim and Konstantin Novoselov “for groundbreaking experiments regarding the two-dimensional material graphene”.
Source: Twitter
At just one atom thick, Graphene is the thinnest material known to man. Graphene is simply a single, 2 dimensional layer (or monolayer) of carbon atoms. And these atoms are tightly bound in a hexagonal shaped formation (on a 2D plane).
But despite being so ridiculously thin, graphene is incredibly strong – about 40 times as strong as diamond and 200 times stronger than A36 structural steel.
But if it is so thin, how is it so strong?
Graphite (the parent of graphene) is naturally a very brittle compound – remember how easily your pencils tip can break? – this is because graphite is made up of many individual, fragments of graphene, separated and unconnected. Graphene, on the other hand, is a single, continuous sheet of carbon atoms. No fragmentation. If you place multiple whole sheets of graphene on top of each other, the result is a VERY strong material.
Two sheets of graphene. Source: Graphenea
In addition, being a single sheet of carbon, graphene is an fantastic conductor of heat and electricity, while the fragmented nature of graphite is not. Graphene is truly a remarkable material – one that is quickly changing the world around us.
For a better explanation of the field of graphene and all of the various applications, watch this video:
This is all very interesting, but what on earth does graphene have to do with Parkinson’s?
Well, groups around the world have already started using graphene in many different ways for Parkinson’s research. For example:
- there are attempts to use graphene to better monitor levels of the chemical dopamine levels which is reduced in the brain of people with Parkinson’s (Click here to read more about this)
- researchers are also hoping to use graphene to deliver medication more efficiently (Click here to read more about this)
- researchers are currently using graphene based products to develop new methods of grow stem cells for cell transplantation in Parkinson’s (Click here to read more about this)
- there are also efforts to make biosensors for Parkinson’s using graphene (Click here to read more about this).
But more importantly, this week a group of researchers published a really interesting report that points towards an amazing new potential use for graphene:
Title: Graphene quantum dots prevent α synucleinopathy in Parkinson’s disease.
Authors: Kim D, Yoo JM, Hwang H, Lee J, Lee SH, Yun SP, Park MJ, Lee M, Choi S, Kwon SH, Lee S, Kwon SH, Kim S, Park YJ, Kinoshita M, Lee YH, Shin S, Paik SR, Lee SJ, Lee S, Hong BH, Ko HS.
Journal: Nat Nanotechnol. 2018 Jul 9. [Epub ahead of print]
PMID: 29988049
In this study, the researchers exposed graphene quantum dots to the Parkinson’s-associated protein alpha synuclein.
Hang on a second. What are graphene quantum dots?
Graphene quantum dots are simply defined as graphene sheets with diameter of less than 30 nanometers ( a nanometer is one-billionth of a meter).
Graphene quantum dots. Source: RSC
OK. And remind me again, what is alpha synuclein?
Here at the SoPD, we often talk about this one particular protein called alpha synuclein. It is one of the most common proteins in the human brain, and it appears to be centrally involved with Parkinson’s.
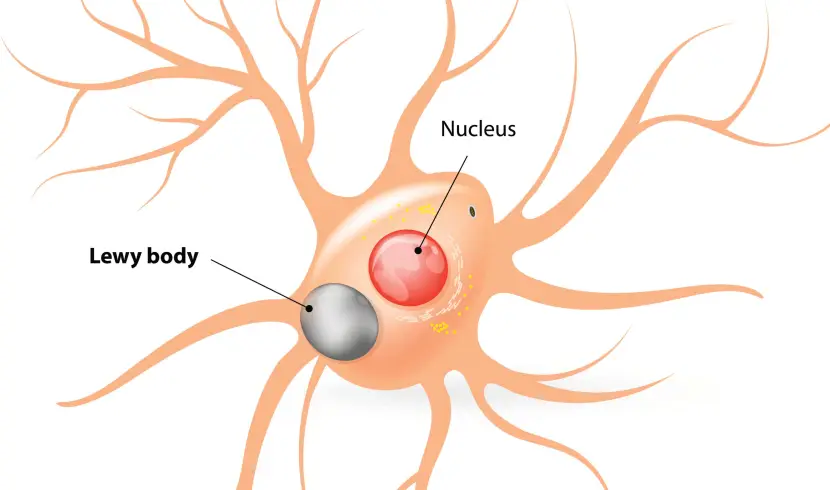
In the Parkinsonian brain, alpha synuclein clumps (or aggregates) together, which is believed to lead to the appearance of Lewy bodies.
What are Lewy bodies?
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells. In the image below, alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (stained in brown; indicated by arrows). Source: Wikimedia
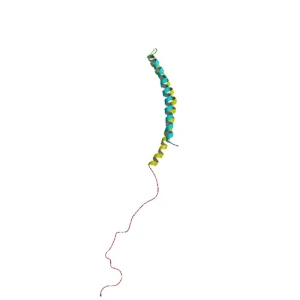
When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure. When it is first produced, alpha synuclein will look something like this:
Alpha synuclein. Source: Wikipedia
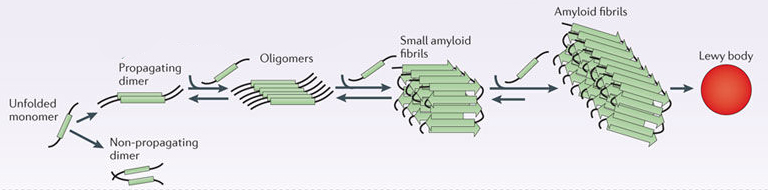
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of Monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein give rise to the aggregations of protein that go on to form the Lewy bodies we mentioned above:
Parkinson’s associated alpha synuclein. Source: Nature
Now, given this process, and its association with a neurodegenerative condition like Parkinson’s, a lot of effort is being put into reducing this aggregation of alpha synuclein protein. It is hoped that by limiting this activity, we may be able to slow or stop completely the progression of the condition.
So what happened when the researchers exposed alpha synuclein to graphene quantum dots?
When the researchers exposed alpha synuclein to the graphene quantum dots in solution (at 37 degrees), the graphene quantum dots stopped the monomeric alpha synuclein from forming into fibrils.
In other words, there was no aggregation.
Wow!
Wow indeed.
But wait, it gets better.
Not only did graphene quantum dots stop the aggregation of monomeric alpha synuclein, but they also caused preformed/mature alpha synuclein fibrils to dissociate into short fragments. After 3 days in solution together, there were less of these fragments, and by 7 days the fragments could not even be detected.
These results were generated by looking at alpha synuclein and graphene quantum dots in solution, so the researchers next wanted to assess whether the same outcome occurred in cells.
And? Did it also occur in cells?
Yes, when the researchers treated neurons grown in cell culture to pre-formed alpha synuclein fibrils they found that alpha synuclein in the cells would begin to aggregate and the cells would die. But when they exposed the neurons to graphene quantum dots, they observed a significant reduction in the aggregation of alpha synuclein protein and the cells survived.
Amazing.
But wait, it gets even more interesting.
Next, the investigators wanted to assess whether these graphene quantum dots could also do this same trick inside the brain. We often see many interesting phenomenon in cell culture that do not translate into the brain. So the researchers started some experiments in mice.
Source: PBS
The researchers injected preformed fibrils of alpha synuclein into the mice. In mice, these fibrils start to cause aggregation, and after one month alpha synuclein protein aggregation can be observed in the brain. The mice start to exhibit motor problems 3-6 months later. In this study, the researchers treated half the mice with graphene quantum dots every two weeks, for 6 months.
They reported that the graphene quantum dots treatment was well tolerated by the mice, but it also significantly reduced the amount of protein aggregation in the brain AND reduced the level of neurodegeneration occurring (which was confirmed by better behavioural/motor scores).
And to further validate the graphene quantum dots treatment in mice, the investigators conducted a second study on hA53T genetically engineered mice.
And what are hA53T genetically engineered mice?
There is a region of your DNA that provides the instructions for making alpha syncuclein. That region of DNA is called SNCA. And there are several genetic variations (or mutations) inside of SNCA that are associated with an increased risk of developing Parkinson’s.
A53T is the name of one of those genetic variations.
As you can see in the image below, A53T lies in the red (Amphipathic) region of SNCA along with several other genetic variants, such as A30P and E46K:
Mice have been genetically engineered to carry the SNCA gene with the A53T genetic mutation (Click here to read the original report). These mice exhibit hyperactivity and then start to display signs of alpha synuclein protein aggregation at about four to six months of age. They also pass away earlier than normal mice (12-14 months of age, compared to 20+ months for normal mice).
In this study, the researchers started treating the hA53T mice with graphene quantum dots every two weeks from 6 months of age. The mice were treated and monitored for 4 months, and the investigators reported a significant reduction in levels of alpha synuclein aggregation in the brains of the treated mice (compared to the non-treated, control hA53T mice).
Importantly, the researchers also mentioned that “that there is no loss of dopamine neurons, glial
cells activation, behavioural abnormalities and organ damage in mice with 6 months of prolonged graphene quantum dots injection, demonstrating that graphene quantum dots manifest no appreciable long-term in vitro and in vivo toxicity and can be cleared from the body and excreted into urine”
Amazing. Is this the first time anyone has used graphene against alpha synuclein?
As far as I’m aware, yes (and I am happy to be corrected on that).
There is some evidence to suggest that graphene quantum dots have no effect on some of the classic neurotoxin-models of Parkinson’s (Click here to read more about this), which is interesting.
But the report reviewed in this post today is not the first time this effect has been observed in other proteins that like to aggregate.
Several years ago, some Chinese researchers reported a similar result when they exposed graphene quantum dots to beta amyloid.
What is beta amyloid?
Beta amyloid is to Alzheimer’s, what alpha synuclein is to Parkinson’s. It is a protein that results from enzymes cutting up the Amyloid precursor protein, and aggregated clusters of beta amyloid (called plaques) are found in the brains of people with Alzheimer’s.
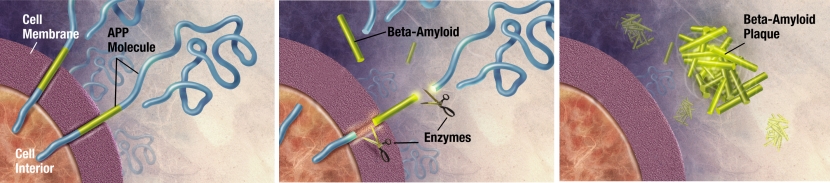
The releasing of Beta Amyloid. Source: Wikimedia
As with alpha synuclein-containing Lewy bodies in Parkinson’s, these beta amyloid-containing plaques are believed to be involved in the neurodegenerative process of Alzheimer’s.
And in 2015, researchers investigating beta amyloid and graphene quantum dots published this report:
Title: Graphene quantum dots for the inhibition of β amyloid aggregation.
Authors: Liu Y, Xu LP, Dai W, Dong H, Wen Y, Zhang X.
Journal: Nanoscale. 2015 Dec 7;7(45):19060-5.
PMID: 26515666
In this study, the researchers exposed graphene quantum dots to beta amyloid protein and they found that it prevented aggregation and also blocked the cytotoxicity of the aggregated protein in cells. In a comparison of six known inhibitors for beta amyloid aggregation, graphene quantum dots were found to have the lowest toxicity levels in cells grown in culture.
But how is this possible? What is the anti-aggregation mechanism of graphene quantum dots?
It is not entirely clear yet, but the researchers point out that both alpha synuclein and beta amyloid have hydrophobic cores. That is to say, both proteins repel water. And they also note that many of the carbon nanomaterials that have demonstrated beta amyloid inhibitory properties, also share a hydrophobic nature. Thus, this hydrophobia may be a shared feature that could be driving the anti-aggregation behaviour.
But this need further investigations.
So where can I get me some of this…
Before you finish that question, it is important to remember that this research is EXTREMELY experimental.
There have never been any registered clinical trial for graphene quantum dots that I am aware of (and I will be happy (and slightly disturbed) to be corrected on this).
Other types of nano dots have been injected into primates in pre-clinical testing of toxicity, and the results of those studies have been telling. For example:
Title: A pilot study in non-human primates shows no adverse response to intravenous injection of quantum dots.
Authors: Ye L, Yong KT, Liu L, Roy I, Hu R, Zhu J, Cai H, Law WC, Liu J, Wang K, Liu J, Liu Y, Hu Y, Zhang X, Swihart MT, Prasad PN.
Journal: Nat Nanotechnol. 2012 May 20;7(7):453-8.
PMID: 22609691
In this study, the researchers intravenously injected a different type of quantum dot (phospholipid micelle-encapsulated quantum dots) which they found to be very well tolerated. Blood and biochemical markers remained did not change over the 90 days of period of observation, and there was no evidence of toxicity in any of the organs analysed.
But it is interesting to note that after 90 days, many of the quantum dots had accumulated in the liver, spleen and kidneys. The researchers concluded that this meant that “the breakdown and clearance of quantum dots is quite slow, suggesting that longer-term studies will be required to determine the ultimate fate of these” quantum dots.
Thus, while quantum dots appear to have few toxicity issues in the short term, there are big questions regarding the clearance of them from the body to be addressed before we start looking at any kind of clinical testing. Until then, we will focus on the clinical testing of other anti-alpha synuclein approaches (Click here and here to read about two examples).
So what does it all mean?
Researchers have identified a new method of reducing the protein aggregation that is associated with Parkinson’s. Given the tiny scale of this new approach – graphene quantum dots – their findings open up a novel class of potential therapeutics for Parkinson’s: Nanomedicines.
I have often invoked a ‘war’ theme in the fight against Parkinson’s on this website.
And this week I can’t help but get caught up in this idea again. It was a long time ago that we made a small beach head landing in the Bay of L-dopa, and initially the progress was slow. But not a week goes by now when there isn’t a new theatre or front line opening up and we are introduced to new weapons being rolled out for testing.
This particular week we got word front the front of a new form of weapon being proposed – a remarkably small, but potentially very powerful weapon. And like the Atomic bombs of previous wars, my sincerest hope is that we will never have to use it. That is to say, by the time it becomes available for use, the war will be over due to all the other weapons under development. But it is also reassuring to know that this marvellous new weapon is being developed and we will have it in reserve if future battles require it.
Ok, enough of the theatrics.
On wards and upwards.
The banner for today’s post was sourced from inhabitat.















Fantastic! These studies are truly amazing, as is the whole breadth of research into a cure. Really gives me hope. Thank you for the enlightening and vastly engaging post.
LikeLike
Hi Tom,
Glad you liked the post – it is amazing the breadth of approaches being applied. And given the popularity of graphene at the moment, I would not be surprised if this particular area receives a LOT of attention.
Kind regards,
Simon
LikeLike
By the way. We’re the mice exposed to the quantum dots through blood, or CSF? Do they cross the BBB (so small, I can guess the answer). I wonder if the quantum dot nature is significant, or perhaps could a roll of graphene be inserted into the spine or brain, alleviating the potential buildup of dots. If it’s a catalyst effect this may have merit.
LikeLike
Hi Tom,
The mice were injected intraperitoneally with the graphene quantum dots and they had no problems crossing the blood brain barrier (determined using both an in vitro assay and in vivo analysis). It will be interesting to see if the researchers can use an intravenous approach to increase the amount getting into the brain – avoiding build up in the other organs and perhaps lowering the required dose.
Regarding a roll of graphene in the spine: Interesting idea – beyond me, but interesting idea.
Kind regards,
Simon
LikeLike
As a Materals Scientist I am amazed at the uses to which graphene is put.
I was womdering if a graphene filter for blood external to the body (in the manner of platelet collection) would work [to avoid the injection of foreign matter into the body; could speed the application.]?
Keith
LikeLike
Hi Keith,
Great question – beyond me, but great question.
Kind regards,
Simon
LikeLike
Graphene is easy to make in the kitchen. See the youtube channel of Robert Murray-Smith.
LikeLike
Hi John,
It is amazing how easy it is to make graphene now considering how long it took to isolate it the first time. Wondrous stuff, massive potential.
Kind regards,
Simon
LikeLike
Simon,
Two questions about specificity, if I may?
Immediately related to this post, what has the alpha-synuclein done to turn the quantum dots against it so specifically? It isn’t as though the graphene can have a particular shape that docks with the a-s, triggering deconstruction in some way. One would expect the dots, being so elemental, as it were, to be dangerous to many compounds or to none, not just to one we happen to be interested in!
Much more generally, it surprises me somewhat that there is rarely any comment in your, or other research summaries I read, on the specificity of this condition’s confinement to dopamine-producing neurones. Is alpha-synuclein specific to this category of cells? No, emphatically not, as you often remark on how this is one of the commonest proteins floating about in the many kinds of cells of the brain; a protein with many callings. What then, is it specifically about the dopamine cells that predisposes them to this family of disorders? The answer to this question must be staring me in the face!
Your posts are superb, and an enormously generous contribution to the wellbeing of your readers; thank you!
Christopher
LikeLike
Hi Christopher,
Thanks for your comment and interesting question – glad you liked the post.
To your question regarding “what alpha synuclein has done to turn the quantum dots against it so specifically”, as we discussed in the post this is not entirely clear yet. It is still very early days with this quantum dot research, but the researchers do point out that both alpha synuclein and Alzheimer’s-associated beta amyloid have hydrophobic cores (which means that they both repel water). And they also note that many of the carbon nanomaterials that have previously demonstrated anti beta amyloid aggregation properties, also share a hydrophobic nature. Thus, this hydrophobia may be the common feature that could be driving the anti-aggregation behaviour of the quantum dots in some way (exactly how it yet to be addressed). If this hydrophobia property is ‘a thing’ it would be interesting to research this role of water molecules in the aggregation of protein further.
And as to what it is “specifically about the dopamine cells that predisposes them to this family of disorders”: If the answer is staring you in the face, grab it and I’ll recommend you to the Sweds for a Nobel medal! This is the question that has been plaguing PD researchers for decades. And the problem gets even more complicated when you have a closer look at the brain. For example, the dopamine neuron populations of the substantia nigra are severely depleted at the time of diagnosis (~60%), but the immediately neighbouring VTA population of dopamine neurons is much less affected (not until the late stages of the condition). And Parkinson’s/alpha synuclein aggregation is certainly not just a dopamine neuron problem. There is a theory of progression in Parkinson’s called ‘Braak staging’ (https://en.wikipedia.org/wiki/Braak_staging) which highlights many of the different populations that are affected by Parkinson’s – some of them before the motor features really start to appear (such as the dorsal motor nucleus of the vagus nerve in the brain stem).
Prof James Surmeier of Northwestern University offers one interesting theory (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5564322/) which is partly underlying the Isradipine/Steady-PDIII clinical study that is ongoing (https://steadypd3.com/, we also discuss that trial in this post: https://scienceofparkinsons.com/2018/05/01/calcium/)
Sorry my answers can’t provide more answers for you, but if I had all the answers we wouldn’t be here looking for answers I guess. Thanks again for the interesting questions!
Kind regards,
Simon
LikeLike