|
# # # # Alpha synuclein is considered to be an influential factor in Parkinson’s. It is a protein that accumulates and clumps together inside certain nerve cells in many cases of Parkinson’s. Recently, clinical trials have attempted to target alpha synuclein that is floating around outside of cells. Some of the strategies focus on an approach called ‘immunotherapy’, which involves boosting the immune system to help remove the toxic form of this protein from the body. This week, one biotech company – AC Immune – bought the Parkinson’s-associated immunotherapy assets off another biotech company – AFFiRiS – which has been developing a potential vaccine for Parkinson’s. In today’s post, we will discuss what immunotherapy is, look at how AFFiRiS has been trying to apply it to Parkinson’s, and review what AC-Immune plans to do next. # # # # |
AC Immune is a a Switzerland-based biotech company that was foundered in 2003.
They are focused on “improving the lives of patients suffering from Alzheimer’s and other neurodegenerative diseases” (Source).
Their approach has primarily centered around the development of immunotherapy approaches. And this week they made a very interesting announcement.
What is immunotherapy?
Immunotherapy is a method of boosting the body’s immune system to better fight a particular disease.
It involves artificially altering the immune system to target a particular protein/disease-causing agent that is not usually recognised as a pathogen (or a disease-causing agent).
 Immune cells attacking a cancer cell. Source: Lindau-nobel
Immune cells attacking a cancer cell. Source: Lindau-nobel
It is potentially a very powerful method for treating a wide range of medical conditions, and the research on immunotherapy is particularly robust in the field of oncology (‘cancer’). Numerous methods of immunotherapy have been developed for cancer – teaching the immune system to target cancer related markers – and they are currently being tested in the clinic (Click here to read about the many clinical trials now under way).
Interesting, but how is immunotherapy being used to neurodegenerative conditions like Parkinson’s?
One of the big theories about how Parkinson’s progresses involves the idea that a toxic form of the Parkinson’s-associated protein, alpha synuclein, could be being passed from cell to cell.
And as this toxic version of alpha synuclein is absorbed by each new healthy cell, it starts causing trouble in that healthy cell and this results in clustering (or aggregation) of protein, which is believed to lead to the appearance of Lewy bodies in those previously healthy cells.
What are Lewy bodies?
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
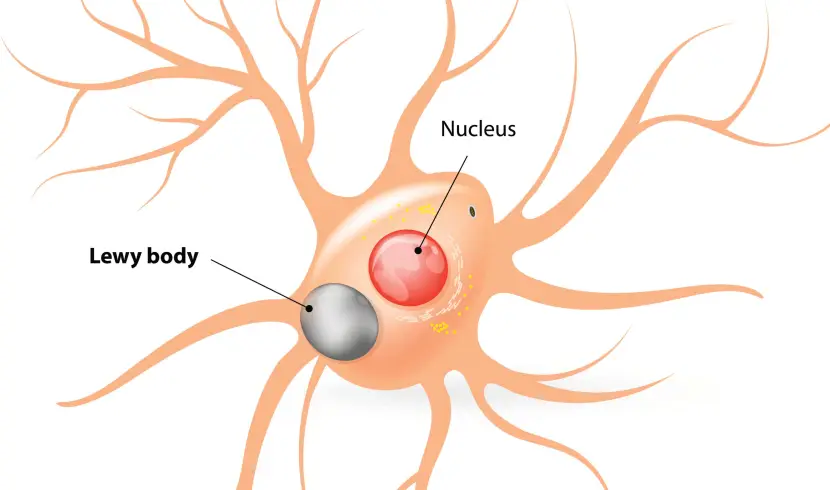 A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells – see the image below where alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (indicated by arrows). Source: Wikimedia
This aggregated form of alpha synuclein is believed to be toxic, which may be causing the loss of certain neurons in the brains of people affected by Parkinson’s. And it is this aggregated form of alpha synuclein that some researchers believe is being passed from cell to cell, seeding Lewy body pathology as it is being passed on.
Is there any evidence that alpha synuclein protein can be transferred between cells?
Back in the 1990s, there were a series of clinical trials of cell transplantation conducted on people with Parkinson’s. The idea was to replace the cells that have been lost to the condition (Click here to read a previous post about cell transplantation). Many of the individuals who were transplanted have now passed away by natural causes and their brains have been examined post-mortem.
One very interesting finding from the analysis of those brains is that some of the cells in the transplants have Lewy bodies in them (up to 10% of transplanted cells in one case – Click here to read the research report on that case).
 Above are photos of neurons from the post-mortem brains of people with Parkinson’s that received transplants. White arrows in the images above indicate Lewy bodies inside transplanted cells. Source: The Lancet
Above are photos of neurons from the post-mortem brains of people with Parkinson’s that received transplants. White arrows in the images above indicate Lewy bodies inside transplanted cells. Source: The Lancet
This finding suggested to researchers that somehow this neurodegenerative condition is being passed on from the Parkinson’s affected brain to the healthy transplanted cells.
And researchers have proposed that the toxic form of the Parkinson’s-associated protein alpha synuclein may be the guilty party in this cell-to-cell transfer process. Being passed from one cell to another and seeding further protein aggregation as they get passed along (Click here to read more on this idea and the evidence for it).
So how is immunotherapy being applied to Parkinson’s?
One way of dealing with this problem of cell-to-cell transfer of the toxic form of alpha synuclein is to grab it as it is being passed between the cells, and remove it from the body. Think of it as vacuuming up all of the toxic form of alpha synuclein that is flowing around outside of cells.
This idea has given rise to a series of ongoing immunotherapy clinical trials that are using antibodies which target the toxic form of the alpha synuclein protein.
What are antibodies?
Antibodies are Y-shaped proteins that the immune system naturally and continuously produces to identify anything in the body that is ‘not self’ (that is, not a normally occurring part of you – think of viruses, bacteria, etc).

Monoclonal antibodies. Source: Astrazeneca
Antibodies act like alert flags for the immune system. When antibodies bind to something, they alert the immune system to investigate and potentially remove the bound object. Each antibody targets a very specific structure, while ignoring everything else.
In this fashion, antibodies are a very powerful method of removing items from the body that are causing trouble or not wanted. And researchers have adapted this natural system for Parkinson’s using immunotherapy approaches.
Currently, immunotherapy is being tested in Parkinson’s in two ways:
- Active immunisation – this approach involves the body’s immune system being encouraged to target the toxic form of alpha synuclein. The best example of this is a vaccine – a tiny fragment of the troublesome pathogen (such as the toxic form of alpha synuclein) is injected into the body before the body is attacked, which helps to build up the immune systems resistance to the pathogen (thus preventing the disease from occurring).
- Passive immunisation – this approach involves researchers designing antibodies themselves that specifically target a pathogen (such as the toxic form of alpha synuclein, while leaving the normal version of the protein alone). These artificially generated antibodies can then be injected into the body.
 Immunotherapy. Source: Acimmune
Immunotherapy. Source: Acimmune
|
# RECAP #1: Many cases of Parkinson’s are associated with the clustering (or aggregation) of a protein called alpha synuclein. Researchers have proposed that the condition progresses by this aggregated form of the protein being passed from one cell to the next. Immunotherapy represents one method by which the aggregated form of alpha synuclein protein can be removed (during cell-to-cell transfer). Tiny proteins called antibodies can be generated to target the rogue form of alpha synuclein, and alert the immune system to remove the offending protein. # |
Ok, so AC Immune have been developing immunotherapies for neurodegenerative conditions. What was their big announcement this week?
The company announced that they would be buying the Parkinson’s-associated immunotherapy assets from an Austrian biotech company called AFFiRiS.
What is so special about the immunotherapy assets of AFFiRiS?
The company has been developing an active form of immunisation – in the form of a vaccine that targets alpha synuclein.
What exactly is a vaccine?
A vaccine is a biological preparation that contains an agent that closely resembles a disease-causing pathogen (virus, bacteria, protein, etc). It is typically just a harmless tiny portion of the pathogen (such as the empty shell of a virus, with none of its disease causing content inside). Sometimes a vaccine contains a weakened or dead form of the pathogen.
The goal of inoculation with a vaccine is to stimulate the body’s immune system to generate antibodies that will target the pathogen in question.
 Vaccination. Source: WebMD
Vaccination. Source: WebMD
In the case of AFFiRiS, the vaccine (called PD01A) has been designed to target the aggregated form of alpha synuclein we were discussing above. An ambitious goal.
Got it. How was AFFiRiS doing with this ambitious goal?
About a year ago, AFFiRiS published all of their Phase 1 clinical trial results of PD01A. The results were published in the prestigous medical journal Lancet Neurology:
 Title: Safety and immunogenicity of the α-synuclein active immunotherapeutic PD01A in patients with Parkinson’s disease: a randomised, single-blinded, phase 1 trial.
Title: Safety and immunogenicity of the α-synuclein active immunotherapeutic PD01A in patients with Parkinson’s disease: a randomised, single-blinded, phase 1 trial.
Authors: Volc D, Poewe W, Kutzelnigg A, Lührs P, Thun-Hohenstein C, Schneeberger A, Galabova G, Majbour N, Vaikath N, El-Agnaf O, Winter D, Mihailovska E, Mairhofer A, Schwenke C, Staffler G, Medori R.
Journal: Lancet Neurol. 2020 Jul;19(7):591-600.
PMID: 32562684
In this report, the researchers outlined the results of their Phase I safety/tolerability studies for PD01A in individuals with recently diagnosed Parkinson’s. I write ‘studies’ because the Phase I project involved 3 consecutive study extensions, resulting in most of the participants being followed for more than 3 years.
The first study involved 24 individuals (45–65 years of age; all no more than 4 years since diagnosis) being recruited before February 2013. They were randomly assigned to one of two treatment groups based on dose:
- 15ug of PD01A (n=12)
- 75ug of PD01A (n=12)
They were given four ‘priming’ injections of PD01A (at baseline, 4, 8, & 12 weeks into the study) and then they were followed out to 52 weeks with assessments, before starting a further 39 week follow up period.
 Source: Alzheimersweekly
Source: Alzheimersweekly
After this initial study, an extension study was initiated that involved a re-randomisation of the participants (to either the 15ug or 75ug group) and a booster shot (another injection of the vaccine). The participants were then followed for a further 24 weeks of assessments.
And after this, the participants were invited to take part in a third extension study, which involved another booster shot, but this time they all received the 75ug injection and were followed for a further 52 weeks assessments.
As far as Phase I studies go, this was rather long. But AFFiRiS – obviously aware of the safety issues with a first-in-human vaccine study – wanted to be safe and thorough.
And what did the results suggest?
Firstly that the treatment is safe and well tolerated in the participants.
In addition, the vaccine also caused the immune system to start producing alpha synuclein targeting antibodies.
Did they reduce alpha synuclein levels in the body?
The concentration of total alpha synuclein protein in the cerebrospinal fluid (the liquid that the brain sits in) did not change as a result of the vaccine treatment. But the researchers reported that at 26 weeks into the study, they observed a 51% reduction in cerebrospinal fluid levels of aggregated alpha synuclein in the 75ug group.
The aggregated form of alpha synuclein makes up just a small percentage of the total levels of alpha synuclein – hence the reason there was no reduction in total levels.
How long did the vaccine effect last?
The researchers found that two years into the study, the levels of alpha synuclein targeting antibodies had dropped back to near baseline levels, but the investigators found that the immune system could be rapidly reactivated after booster shots of the vaccine.
And what did the vaccine do for the Parkinson’s symptoms of the study participants?
Ok, so here we need to be really careful with what we take away from the report.
It is important to remember that this is an open label study – meaning that all of the participants knew what they were being treated with. In such situations there is an increased risk of having a placebo response to a treatment (this is a situation where an individual experiences benefits that may have no biological association with the treatment being administered).
Placebo responses are an issue in Parkinson’s trials as individuals with the condition appear to be more prone to experiencing them (Click here to read an old SoPD post on this topic).
In addition, we have to be careful in managing expectations. We do not want to be raising expectations, if in the long term a treatment does not go on to be approved by health regulators.
So, with all of that said: Regarding assessments of disease progression, the researchers wrote in their report that:
“DAT-SPECT examinations did not show statistically significant changes up to 91 weeks in study 1. MDS-UPDRS part 3 scores were generally stable across the studies”
What does any of that actually mean?
Two important details here:
- DAT-SPECT is a type of brain imaging technique (specifically, it is a type of single-photon emission computed tomography (SPECT) imaging technique that allows for the visualisation of dopamine transporter (DAT) levels in the brain). Dopamine transporter is a protein on the membrane of dopamine neurons involved in dopamine turnover. By labelling DAT, one can get an indication of how many dopamine terminals there are in the brain.
- The Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) is the most commonly used clinical assessment in clinical trials. Part 3 of the scale focuses on the motor features of the condition. There are 33 items assessed (in effect there is only 18 items, but several address which side of the body, or which limb is affected). An increase in UPDRS score indicates progression of Parkinson’s features, while a lower score suggests correction or improvement (Click here to read a recent SoPD post about the MDS-UPDRS).
Now remembering that this is just a small open-label study not designed to test efficacy of the vaccine, the researchers were reporting that they saw no deterioration in DAT levels (according to the DAT-SPECT brain imaging), and no change in clinical assessments of motor symptoms across multiple years of assessment.
They state “Mean MDS-UPDRS part 3 scores in the pooled 15 μg group were 11·9 (SD 8·2) at baseline and 12·5 (14·8) at the last visit. In the pooled 75 μg group, MDS-UPDRS part 3 scores were 12·3 (7·2) at baseline and 8·6 (7·7) at the last visit”
And they added that this stablisation of the clinical features of Parkinson’s was “in contrast to previously published data which reports a worsening in MDS-UPDRS part 3 scores in a similar population of patients with early Parkinson’s” (they cite this reference to support this statement).
So the vaccine slowed progression?
While the results may appear encouraging, please remember that this was a small, open label Phase 1 study not designed to test efficacy. Interpretation of the results must be handled with great caution. A larger, double-blind Phase 2 double blind study is what is really required to determine if there is any evidence of efficacy.
Currently all we can really take away from this study is that the treatment was safe and well tolerated in individuals with Parkinson’s over multiple years.
And the researchers acknowledge this in their concluding statement: “the safety profile and positive antibody response of PD01A supports the further development of this immunotherapeutic for the treatment of Parkinson’s disease in a phase 2 clinical trial“.
So is there going to be a Phase 2 study?
In January of 2020, AFFiRiS announced that based on feedback from the US FDA, they will proceed with preparations for a Phase 2 clinical. They planned to initiation the study in the US and Europe in the second half of 2020 (Click here to read more about this).
But then 2020 got kind of disrupted by a little thing called COVID-19.
|
# # RECAP #2: Austrian biotech company AFFiRiS has been developing an alpha synuclein-targeting vaccine for Parkinson’s. The company has previously published the results of their Phase 1 clinical trial of their lead vaccine (called PD01A) is 24 people with recently diagnosed Parkinson’s over multiple years. The results indicate that the treatment was safe and well tolerated. In addition, it caused the immune system to generate antibodies against aggregated alpha synuclein protein, and this effect lasted for approximately 2 years. # # |
So AC Immune has purchased all of AFFiRiS’s “Parkinson’s vaccine” assets?
In their press release, AC Immune announced that they were “acquiring all of AFFiRiS’ assets and underlying intellectual property related to active vaccine candidates targeting a-syn and USD 5 million in cash for 7.1 million shares based on a price of USD 8.26 per common share. This share price represents a 10.7% premium compared to the closing price of AC Immune shares as of July 23, 2021″.
The company also stated that they would be immediately launching “clinical development of ACI-7104, the optimized formulation of PD01, into an adaptive, biomarker-based Phase 2 study”.
Very little is currently known about this new trial except that “This trial will evaluate an initial dose-response of the optimized formulation focusing on immunogenicity against a-syn and pathological a-syn species. Additionally, progression of motor and non-motor symptoms of Parkinson’s disease will be monitored, together with digital, imaging and fluid biomarkers”.
What is meant by “adaptive”?
Adaptive clinical trials have a predefined level of flexibility baked into their design. The data is being continually monitored in adaptive trials and certain aspects of the study can be changed as it progresses (such as increasing dose or terminating a particular arm that is displaying no treatment effect). If you are interested in reading more about adaptive clinical trials – click here to read a previous SoPD post on this topic.
Sounds interesting. Why do you think AC Immune is making this purchase?
The company has had an interest in alpha synuclein immunotherapy for some time, and recent activities suggest that this interest is only getting stronger.
Two days after the announcement AC Immune made a poster presentation at the Alzheimer’s Association International Conference (AAIC) 2021. The poster presented ACI-12589 – a first-in-class positron emission tomography (PET) imaging tracer targeting alpha synuclein.
The company reported preclinical data confirming that ACI-12589 was able to access the brain in non-human primates. In addition, they presented data demonstrating ACI-12589’ showed great specificity for alpha synuclein on tissue samples from patients with alpha-synucleinopathies, including Parkinson’s, multiple system atrophy (MSA), and dementia with Lewy bodies (DLB).
If this imaging tool works in patients, it could represent a very powerful tool for monitoring alpha synuclein activity in the brains of people with Parkinson’s. It would also be extremely useful in a clinical trial assessing a vaccine for Parkinson’s.
AC Immune appears to be laying the foundation for a significant bet on alpha synuclein targeting approaches in Parkinson’s.
Is AC immune (AFFiRiS) the only biotech company developing a vaccine for Parkinson’s?
There are multiple companies developing alpha synuclein-targeting immunotherapies for Parkinson’s (we have previously discussed the “Pasadena study” which is the most advanced – click here to read that post). Most of these experimental treatments, however, are “passive” forms of immunotherapy – meaning that antibodies are generated in a lab and then injected into the body on a regular basis.
But there is another biotech company working on a vaccine for Parkinson’s, and that company is called Vaxxinity (previously known as United Neuroscience).
This biotech company is focused on developing a new class of fully synthetic vaccines (they call them ‘endobody vaccines‘). Endobodies have made an impact on animal health with over 3 billion doses being administered in veterinary applications, and United Neuroscience is now shifting this technology to humans.
Their first human clinical trial – of a beta amyloid targeting vaccine called UB-311 in individuals with mild Alzheimer’s – was a Phase II trial, but in December 2019, the company terminated the extension of that trial following a review of the trial data, referring to “the treatment assignment error issue”. I am not sure what that means and no further information has been made available (Click here to read more about that study).
But in 2020, Vaxxinity outlined a plan for a Phase 3 program, involving two identical, double-blind, placebo-controlled, 73-week studies. These parallel trials would enroll 3,218 participants with mild Alzheimer’s. The participants would receive three priming and four booster immunizations (or placebo treatment).
As the image above shows, the company also has an ongoing Phase I trial for their alpha synuclein targeting vaccine UB-313 in Parkinson’s (Click here to read more about that study). The study involves 62 participants (both healthy volunteers and people with Parkinson’s).
The study consists of two parts:
- Part A of the study will focus on healthy volunteers and will consist of a dose escalation investigation for up to seven planned dose levels (or placebo).
- Part B of the study will involve a Parkinson’s cohort and will be based on safety, tolerability and immunogenicity (the response from the immune system).
The study will be a 44-week long, consisting of 20 weeks of treatment and 24 weeks of follow-up. It is expected to conclude in mid 2022.
So what does it all mean?
At the SoPD, we propose that any ‘curative’ therapy for Parkinson’s is going to require three components:
- A disease halting mechanism
- A neuroprotective agent
- Some form of cell replacement therapy
The acquisition of the “vaccine” assets of AFFiRiS by AC Immune represents some encouraging news regarding the one of these components: A disease halting mechanism.
Ten years ago, the idea of a vaccine for Parkinson’s was the stuff of fiction, but Austrian biotech firm AFFiRiS took on the challenge and they should be congratulated for achieving all that they have to date. The Phase 1 results that they published represent an incredible accomplishment both for the company and the researchers involved, but also for the brave participants who have taken part in the long trial.
At a time when there have been a long run of disappointing results surrounding immunotherapy for Alzheimer’s and a raging debate regarding the theories behind this approach for neurodegeneration in general – it is interesting that AC Immune is taking this step towards a vaccine approach. We now need to see the initiation of the larger Phase 2 study that will be designed to indicate whether a alpha synuclein targeting vaccine can slow the progression of Parkinson’s.
Based on the statement in their press release regarding the immediate initiation of “an adaptive, biomarker-based Phase 2 study“, we hopefully won’t have to wait too long.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from AC Immune & AFFiRiS






5 thoughts on “AC Immune acquires assets of AFFiRiS”