|
Trehalose is a small molecule – nutritionally equivalent to glucose – that helps to prevent protein from aggregating (that is, clustering together in a bad way). Parkinson’s disease is a neurodegenerative condition that is characterised by protein aggregating, or clustering together in a bad way. Is anyone else thinking what I’m thinking? In today’s post we will look at what trelahose is, review some of the research has been done in the context of Parkinson’s disease, and discuss how we should be thinking about assessing this molecule clinically. |
Neuropathologists examining a section of brain tissue. Source: Imperial
When a neuropathologist makes an examination of the brain of a person who passed away with Parkinson’s, there are two characteristic hallmarks that they will be looking for in order to provide a definitively postmortem diagnosis of the condition:
1. The loss of dopamine producing neurons in a region of the brain called the substantia nigra.
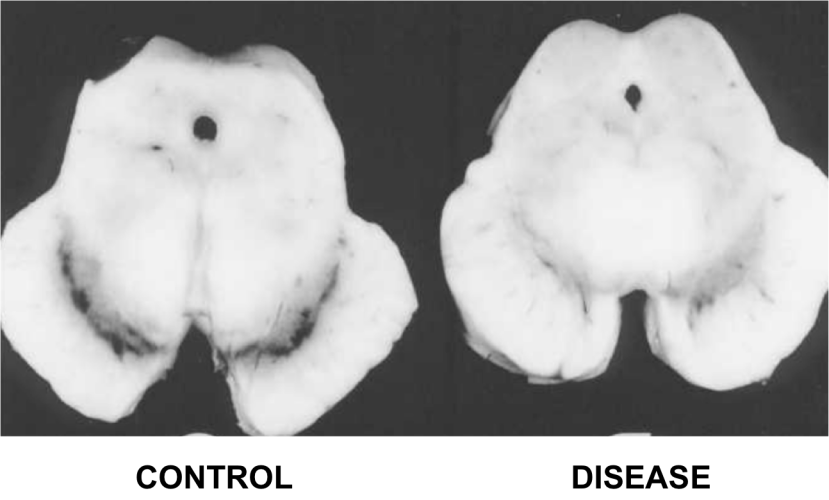
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinson’s disease brain (right). Source:Memorangapp
2. The clustering (or ‘aggregation’) of a protein called alpha synuclein. Specifically, they will be looking for dense circular aggregates of the protein within cells, which are referred to as Lewy bodies.
A Lewy body inside of a neuron. Source: Neuropathology-web
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
In addition to Lewy bodies, the neuropathologist may also see alpha synuclein clustering in other parts of affected cells. For example, aggregated alpha synuclein can be seen in the branches of cells (these clusterings are called ‘Lewy neurites‘ – see the image below where alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s disease.
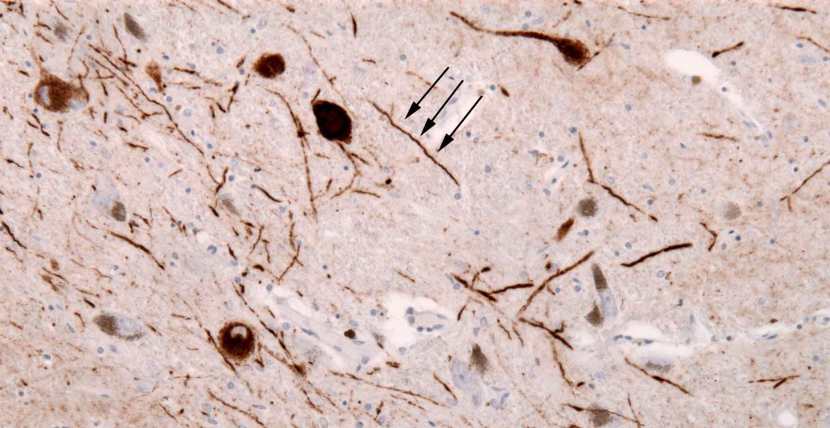
Examples of Lewy neurites (indicated by arrows). Source: Wikimedia
Given these two distinctive features of the Parkinsonian brain (the loss of dopamine neurons and the aggregation of alpha synuclein), a great deal of research has focused on A.) neuroprotective agents to protect the remaining dopamine-producing neurons in the substantia nigra, and B.) compounds that stop the aggregation of alpha synuclein.
In today’s post, we will look at the research that has been conducted on one particular compounds that appears to stop the aggregation of alpha synuclein.
It is call Trehalose (pronounces ‘tray-hellos’).
What is Trehalose?
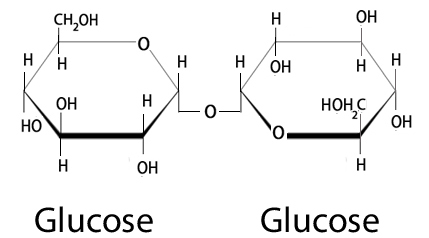
Trehalose (also known as alpha-D-glucopyranosyl-1,1-alpha-D-glucopyranoside) is a disaccharide.
What is a disaccharide?
A disaccharide is a molecule that is composed of two smaller glucose molecules linked together.
Trehalose – 2 molecules of glucose. Source: Nutrientsreview
If that simple definition of a disaccharide is too basic for you and you want something more thorough, try this:
(This guy – Andrey Kopot of AK Lectures – is amazing!)
Trehalose can be found (in small amounts) in mushrooms, honey, lobsters, shrimps, certain seaweeds, wine, beer (don’t get too excited!), and most foods produced using baker’s yeast. And it is generally considered safe nutritionally – the U.S. Food and Drug Administration lists trehalose as a compound under the category of GRAS (or ‘Generally Recognised as Safe’) (Source and Source).
Trehalose occurs naturally in a wide variety of organisms, ranging from bacteria to invertebrates, and it serves as an energy source or stress protectant. For an interesting OPEN ACCESS review of the biological functions of trehalose – Click here.
And trehalose stops alpha synuclein from aggregating?!?
Yes.
Back in 2004, this research report was published:

Title: Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease.
Authors: Tanaka M, Machida Y, Niu S, Ikeda T, Jana NR, Doi H, Kurosawa M, Nekooki M, Nukina N.
Journal: Nat Med. 2004 Feb;10(2):148-54.
PMID: 14730359
Huntington’s disease is another neurodegenerative condition that is characterised by the aggregation of protein.
In the case of Huntington’s, however, the protein is called huntingtin (not a typo, that is the protein’s name). The researchers who conducted this study wanted to identify compounds that could reduce the aggregation of huntingtin protein, so they conducted a large screening experiment (more than 200 compounds) on cells growing in culture. They found that disaccharides appear to share an ability to slow the aggregation of huntingtin protein. One disaccharide in particular displayed really significant results… take a wild guess which one!
When the researchers feed trehalose (2% in drinking water – that is 2 grams trehalose in 100ml water) to mice that have been genetically engineered to have aggregation of huntingtin protein, they found that trehalose not only decreased huntingtin aggregates in brain and liver, but it also improved motor function in these mice and extended their lifespan (beyond that of the huntingtin-producing mice that were not treated with trehalose). The researchers concluded that the “lack of toxicity and high solubility, coupled with efficacy upon oral administration, make trehalose promising as a therapeutic drug or lead compound for the treatment” for Huntington’s disease.
But what about alpha synuclein?
Well, this aggregate-inhibiting ability of trehalose got other researchers thinking, and a couple of years later this research report was published:
Title: Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein.
Authors: Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC.
Journal: J Biol Chem. 2007 Feb 23;282(8):5641-52.
PMID: 17182613 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers replicated the findings of the previous study, and they then turned their attention to the Parkinson’s associated protein alpha synuclein and they found that trehalose also inhibits aggregation of this protein as well. What was particularly interesting, however, was their discovery that trehalose as an autophagy activator.
And what is autophagy?
Autophagy is an absolutely essential function in a cell. Without autophagy, old proteins and mitochondria will pile up making the cell sick and eventually it dies. Through the process of autophagy, the cell can break down the old protein, clearing the way for fresh new proteins to do their job.
Think of autophagy as the waste disposal/recycling process of the cell.

The process of autophagy. Source: Wormbook
Waste material inside a cell is collected in membranes that form sacs (called vesicles). These vesicles then bind to another sac (called a lysosome) which contains enzymes that will breakdown and degrade the waste material. The degraded waste material can then be recycled or disposed of by spitting it out of the cell.
By inducing autophagy, the researchers of this study showed that trehalose also protects cells against toxic insults. And this result has since been replicated by several independent research groups (Click here, here, here and here to read more about this).
Trehalose has also been tested in animal models of Parkinson’s, such as this study:

Title: Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressingmice through autophagy activation.
Authors:Rodríguez-Navarro JA, Rodríguez L, Casarejos MJ, Solano RM, Gómez A, Perucho J, Cuervo AM, García de Yébenes J, Mena MA.
Journal:Neurobiol Dis. 2010 Sep;39(3):423-38.
PMID: 20546895
In this study, the researchers looked at the use of trehalose in a genetic mouse model of Parkinson’s disease. These mice produce a great deal of human Tau protein and have no Parkin protein.
What is Tau?
I will be addressing Tau in an upcoming post, but briefly, Tau is a microtubule-associated protein.
What does that mean?
Microtubules are basically the highways and byways inside a cell, and Tau functions by stabilising those highways and byways. Tau holds together the roads which allows stuff to be moved around the cell (usually in sacks called vesicles, by transporter proteins like kinesin).

Tau: a stabilising presence. Source: Hindawi
So why is TAU interesting?
Because it is associated with both Parkinson’s disease AND Alzheimer’s disease. In both conditions there is a build up of Tau protein which accumulates and aggregates in a similar fashion to alpha synuclein (Click here for a good review article on this topic). Thus, by engineering mice to over-produce Tau, the researchers can model aspects of Parkinson’s disease.
Ok, but what about Parkin? What is that?
About 10% of Parkinson’s cases are associated with particular genetic variations that render people vulnerable to developing the condition. Some of these mutations are in sections of DNA (called genes) that provide the instructions for proteins that are involved in the process of autophagy (which I mentioned above). Two genes, in particular, are the focus of a lot of Parkinson’s-related research – they are called Parkin and Pink1.
What do Parkin and Pink1 do?
Both proteins appear to have many different functions, but their roles in the process of autophagy are well understood.
Pink1 acts like a kind of handle on the surface of old proteins that need to be broken down and recycled. And if Pink1 a handle, then Parkin is a flag that likes to hold onto the Pink1 handle. While exposed on the surface of old proteins, Pink1 starts grabbing the Parkin protein. This pairing is a signal to the cell that this particular bit of old protein is rubbish and needs to be removed. In the absence of normal Pink1 or Parkin proteins, there is no handle-flag system and old proteins start to pile up. They are not disposed of appropriately and as a result the cell getting sick and ultimately dying.
People with particular mutations in the Pink1 or Parkin genes are vulnerable to developing an early onset form of Parkinson’s disease. It is believed that the dysfunctional autophagy process is part of the reason why these individuals develop the condition at such an early age.
So the investigators have engineered a mouse that produces lots of Tau, and has no Parkin to help remove the waste?
Exactly.
Poor mouse!
Indeed.
But when they treated those mice with trehalose (1% in fresh drinking water, twice a week for 2.5 months from infancy), the researchers found that they could reduce the dopamine cell loss that is seen in these animals and also decrease the amount of Tau aggregates in the brain. And this neuroprotective process was partly the result of increasing autophagy (there are different mechanisms of autophagy, some of them are independent of Parkin – click here to read more about this).
What is particularly interesting in this study, however, was that after the investigators observed this effect, they next delayed treating the animals until 14-months of age (mid-late age for mice who live 2-2.5 years). They treated these older mice for just 3 weeks with 1% trehalose and observed improvements in motor behaviour, levels of anxiety, and reductions in their levels of aggregated protein in the brain.
Another interesting finding in this study is that while short term use of trehalose resulted in positive effects, consistent longer term use (4 months) of the compound was not associated with improvements in the dopamine system and also had lower body weights than their placebo-treated controls. This long term aspect of using trehalose needs to be further investigated independently. If it is replicated, then sporadic treatment with trehalose will need to be looked at.
This first investigation of trehalose in a model of Parkinson’s disease has subsequently been followed up by other research groups, including this report:

Title: Neuroprotective effect of the chemical chaperone, trehalose in a chronic MPTP-inducedParkinson’s disease mouse model.
Authors: Sarkar S, Chigurupati S, Raymick J, Mann D, Bowyer JF, Schmitt T, Beger RD, Hanig JP, Schmued LC, Paule MG.
Journal: Neurotoxicology. 2014 Sep;44:250-62.
PMID: 25064079
The researchers who conducted this study used a neurotoxin (MPTP) to model Parkinson’s in mice. They found that pre-treatment with trehalose (for 6 weeks, starting 3 days before the neurotoxin was injected) significantly reduced the loss of dopamine neurons. Interestingly, trehalose also reduced the levels of microglial activation in MPTP injected mice, indicating protection against neuroinflammation.
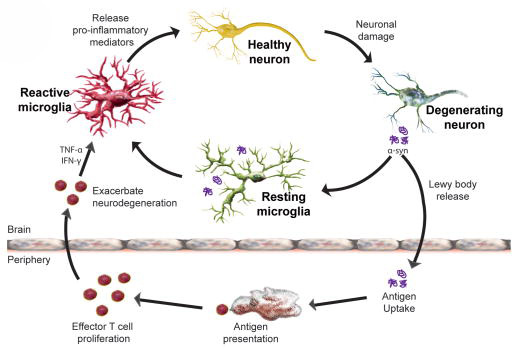
What are microglia? And what is neuroinflammation?
Microglia are cells in the brain that form the main part of active immune defence to injury or disease. When any kind of trouble kicks off in the brain, microglia become activated and start dealing with the source of the trouble as well as removing any damaged/dying cells. They communicate with other cells (particularly cells of the immune system, such a T-cells and macrophage) by releasing chemicals which is part of the process of inflammation.
Microglia become activated around sick cells and release pro-inflammatory compounds. Source: NCBI
By reducing activation of microglia and subsequent inflammation in the brain, trehalose appears to provide further protection to the dopamine neurons.
Another investigation of trehalose in an animal model of Parkinson’s addressed the issue of alpha synuclein:
Title: Treatment with Trehalose Prevents Behavioral and Neurochemical Deficits Produced in an AAV α-Synuclein Rat Model of Parkinson’s Disease.
Authors: He Q, Koprich JB, Wang Y, Yu WB, Xiao BG, Brotchie JM, Wang J.
Journal: Mol Neurobiol. 2016 May;53(4):2258-68.
PMID: 25972237
In this study, the researchers tested the ability of trehalose to protect cells against a mutant version of the alpha synuclein protein in rats. The mutant version of alpha synuclein was inserted into dopamine neurons via a virus, and this transfer caused the cells to slowly die, resulting in behavioural motor problems for the animals. The investigators found that at concentrations of 2% and 5% in drinking water (for 6 weeks; started immediately after the alpha synuclein virus was injected), trehalose protected dopamine cells against the toxic effects of alpha synuclein. In addition, they found that trehalose did a great job of activating autophagy, which helped clear the excess alpha synuclein from the cells.
The investigators concluded that “this study supports the concept of using trehalose as a novel therapeutic strategy that might prevent/reverse alpha synuclein aggregation for the treatment of Parkinson’s disease“. They also suggested that “further investigation is needed to define clinical design strategies and clarify the relationship between autophagy and neuroprotective effect of trehalose“.
Has Trehalose been tested clinically in Parkinson’s disease?
No. Not yet. It does appear to be on the list of things to do though.
Trehalose has been tested in clinical trials for other conditions (Click here, here and here to read more about this) and appears to have a relatively safe profile.
In addition, there is a biotech company that is looking to take a synthetic version of trehalose into clinical trials.
The company is called Junaxo.

Incorporated in 2013, Junaxo is a ‘virtual biotech’ based out of Toronto (Canada). In a future post we will be re-visiting this ‘virtual biotect’ model of drug development, but basically by outsourcing their research work, companies like Junaxo can reduce the capital required it to reach their scientific/clinical goals.
Junaxo has a candidate drug (JNX3001) that is based on trehalose.
In 2013, the company was awarded a Michael J Fox Foundation Therapeutic Pipeline Program grant to assess the “efficacy of trehalose in an alpha-synuclein-based non-human primate model of Parkinson’s (Click here to read more on this). We are still awaiting the publication of those results, but they would hopefully represent the final pre-clinical data before trehalose (or JNX3001) is proposed for clinical trials.
What does it all mean?
So, summing up: Trehalose is an interesting compound that demonstrates remarkable protein aggregation inhibiting properties, in addition to activating autophagy and reducing inflammation in the brain. So remarkable are these properties, in fact, that they almost seem to good to be true…
…and this is the part of this post where I perform my habitual ‘rain on the parade’ piece.
Here’s the thing: When consumed orally, trehalose can be rapidly broken down into two glucose molecules by the enzyme trehalase, which is present in cells along the lining of the gut and also in the liver. Conversion into glucose is necessary for the body, but it is not a good scenario for our needs related to Parkinson’s disease. A comparative study of the effects of trehalose and glucose has been conducted in the Huntington’s disease study mentioned in this post (Click here), and it appears to rule out the possibility that glucose administration is having the same effects.
Thus, there are serious questions regarding how much orally consumed trehalose could potentially reach the brain in humans (and I am yet to find any evidence that it can cross the human blood-brain-barrier – the protective membrane surrounding the brain). Oral trehalose is accessing the blood stream – a double blind clinical trial reported last year that oral trehalose improves arterial and microvascular function in elderly folks (Click here to read more about this study) – but this does not mean that enough is reaching the brain to have beneficial effects.
One biotech company – Bioblast Pharma Ltd (in Israel) – may have a solution to this problem though.
This company is attempting to take trehalose to the clinic for both oculopharyngeal muscular dystrophy (OPMD – a rare genetic muscle disorder with onset during adulthood) and spinocerebellar ataxia type 3 (SCA3 – a condition characterised by progressive problems with movement). Both of these conditions are associated with protein aggregation. But this company is proposing to treat the conditions by injecting trehalose directly into the blood (bypassing the gut completely). They have just finished a phase I clinical trial testing the safety of this intravenous approach, and we are waiting to here the results of that study. If successful, this could be an option with regards to using trehalose in Parkinson’s disease.
As always, I will report back here when new information comes to hand. A lot of people are looking at trehalose for various conditions, so there will certainly be a lot of new information in the near future.
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Wikipedia







Thanks, Simon!
The glucose reminds me of the connection researchers make with PD and metabolites and lipids. It’s hard to pull it all together, but fun trying. Your posts give us plenty to think about (and I love it : )
LikeLike
Hi Beetheaven, glad you liked the post. It is also similar to the mannitol research that is going on at the moment as well (https://scienceofparkinsons.com/2016/12/28/update-mannitol-and-parkinsons-disease/). I will be very interested to see how this all plays out.
Kind regards,
Simon
LikeLike
Hi Simon, can you tell me what the difference is between Trehalose and Mannitol please?
LikeLike
Hi Gerry,
Thanks for the interesting question.
Basically, Trehalose is a sugar (made up of two connected glucose molecules), while Mannitol is a sugar alcohol. To confuse the matter further, sugar alcohols is neither a sugar nor alcoholic. They are sweetners though, which provide fewer calories than sugar and have less effect on blood sugar levels.
Chemically, sugars and sugar alcohols don’t differ so much. Mannitol is HOCH2 (CHOH)4 CH2OH, while sugars have two fewer hydrogen atoms, for example glucose is HOCH2 (CHOH)4 CHO. Is this the sort of answer you were looking for? I hope this helps.
Kind regards,
Simon
LikeLike
Simon
Yet another interesting piece. I was thinking whilst reading it ‘more treatments for rats & mice, nothing for people!’
The final paragraph gives me some hope. When is this likely to result in a treatment being available? 5yrs , 10yrs ?
kind regards
Keith
LikeLike
Hi Peasantfarmer, it really all depends on the Junaxo primate study that has been funded by MJFF (which I mention at the bottom of the post). If that study shows good levels of the compound actually reaching the brain and a beneficial effect in those animals, a phase II clinical trial would hopefully follow very quickly – it would certainly be justified (and that could potentially be within the next 2 years). If not, then the injectable approach proposed by Bioblast Pharma Ltd should be considered – if their completed phase I trial demonstrates safety (and that would be pushing the time frame closer to 5 years). I think if their phase I trial shows a positive safety outcome, a group like MJFF or CureParkinson’s trust should hop on a plane and fly out to Israel for introductory meetings. I don’t like injectables, but if it works…
Glad you liked the post.
Kind regards,
Simon
LikeLike
Holy Good News Batman! I thought I was a lone voice in the wilderness, but I’m excited to see the work being done by Junaxo. I’m slowly catching up on the wealth of information here. Thank you for your work!
Do you have any articles on the possible benefits of combined therapies?
I have SCA1 but am keeping my symptoms at bay. I believe I am achieving this through my combination of therapies including:
Diet – low cal, low carb, mostly vegetarian, high in cruciferous vegetables
Exercise – daily 30 minutes including running, weights, yoga
Supplements – trehalose oral and intranasal, Niagen 500mg, pterostilbene 250 mg, 2 cups green tea, vitamin D
I believe many patients could be helped if only doctors would try to find personalized complete treatment
LikeLike
Hi Joe,
Thanks for your comment. Sorry to hear about your situation, but well done for taking a pro-active/inspired approach towards it – I have a great deal of respect for that. Personalised medicine is slowly becoming more of a feature with Parkinson’s, but as you suggest it is largely left to the folks dealing with the condition to explore it themselves. There is terrible lack of research articles on it at present (though I suspect that this will change shortly).
There are also very few articles exploring combined therapies for PD. In the new year I will be exploring this idea as major shift on the website. I think cancer treatment is certainly showing us the road ahead (I am about to post a discussion about the I-spy clinical trial approach) and we would be foolish not to explore the idea further. Yes, cancer has more of a genetic base to it than PD, but the combination approach is necessary in both conditions I suspect. Researchers should also be encouraged to start combining compounds in their lab-based experiments (eg. testing nilotinib in combination with an antioxidant like Oleuropein). This kind of research is not only important from the stand point of finding better therapeutic combinations, but also determining which compounds clash (for example the way MAO-B inhibitors can clash with anti-depressants). Eventually, folks with PD may be given an immunotherapy approach (to slow the progression of the condition, think the Prothena clinical trial), a neuroprotective approach (to protect and nurture the cells that remain, such as GDNF or a LRRK2 inhibitor), and this would be supplemented by a cell replacement therapy (restoring the lost function with new cells). The ultimate in combination approaches perhaps. This is where things are heading, and this is what we should be preparing for now.
Thanks again for your inspirational comment.
Kind regards,
Simon
LikeLike
Such a lot of valuable and interesting information here. Thanks Simon (& Joe)
LikeLike
You are very welcome Gerry!
LikeLike
Totally confused. What’s useful what’s not? I’m interested in helping myself because my medical team is difficult to access but I’m not sure what I can do help!
LikeLike
Hi Beryl,
Thanks for your comment and question. This is very relevant to everyone. But the simple answer is that it probably varies from person to person. By this I mean that everyone is different and there is not going to be a single silver bullet that helps everyone.
For example, we have previously discussed the widely used supplement NAC (https://scienceofparkinsons.com/2017/07/06/glutathione-getting-the-knack-of-parkinsons-disease/). Many people in the Parkinson’s community use this, but if a person has a genetic variant in their CBS (cystathionine beta synthase) gene, they should not – they will have no benefit from NAC (if anything it may actually make them feel worse!).
And this is where members of the Parkinson’s community needs to be proactive. They need to learn as much as they can about themselves on an individual basis. I hope this helps.
Kind regards,
Simon
LikeLike
Interested in trehalose, but I wonder if the research indicating it may promote the growth of Clostridium difficile is cause for caution. Especially with the growing evidence of a gut connection to PD, I would think it could be cause for some concern. I have read of the alternative of using trehalose based eyedrops in the hope of getting more of the stuff to the brain. Maybe that would avoid any gut bacteria issues?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5984069/
LikeLike