|
Today biotech company Voyager Therapeutics announced an update on their ongoing phase Ib clinical trial. The trial is evaluating the safety and tolerance of a gene therapy approach for people with advanced Parkinson’s. Gene therapy is a technique that involves inserting new DNA into a cell using viruses. In this clinical trial, the virally delivered DNA helps the infected cell to produce dopamine in order to alleviate the motor features of Parkinson’s. In today’s post we will discuss what gene therapy is, review the new results mentioned in the update, and look at other gene therapy approaches for Parkinson’s. |

Source: Baltimoresun
Voyager Therapeutics is a clinical-stage gene therapy company that is focused on treatments for neurological conditions, such as Parkinson’s. Today the company announced an update of their ongoing Phase 1b trial of their product VY-AADC01 (Click here to see the press release).
VY-AADC01 represents a new class of treatment for Parkinson’s, as it is a form of gene therapy.
What is gene therapy?
The gene therapy involves introducing a piece of DNA into a cell which will cause the cell to produce proteins that they usually do not (either by nature or by mutation). The DNA is artificially inserted into cells and the cell’s protein producing machinery does the rest.
Source: Yourgenome
How does gene therapy work?
The introduction of the section of DNA – which provides the instructions for making a particular protein – is usually achieved using carefully engineered viruses. The viruses have had all the disease causing components removed, allowing us to use the virus as an efficient biological delivery system. Viruses by their very nature are very good at infecting cells, so if we remove the disease causing components, what is left is a very effective system of getting whatever you want into a cell.
Taking this approach one step further, we can take sections of DNA that contain the genes (these are the instructions for making proteins) involved with the production of a chemical called dopamine and insert them into our empty virus.

Gene therapy for Parkinson’s. Source: Wiki.Epfl
Dopamine is a chemical in the brain whose levels are badly affected by Parkinson’s. A severe reduction in dopamine levels results in the use of L-dopa as a treatment for Parkinson’s, as it helps to resort dopamine levels. By injecting a virus with dopamine-production associated DNA into the brain, we can produce dopamine in any infected cells (it’s slightly more complicated than that, but you get the basic idea).
So what is the Voyager trial trying to do?
Voyager Therapeutics‘s gene therapy product, VY-AADC01 is an adeno associated virus (or AAV) that carries a piece of DNA which contains the instructions (or a gene) for making a protein called Aromatic L-amino acid decarboxylase (or AADC).

AAV Viruses. Source: HuffingtonPost
And yeah, I know what you are going to ask next:
What is AADC?
AADC is the enzyme involved in the production of the chemical dopamine. Specifically, AADC converts the chemical L-dopa into dopamine. L-dopa is naturally produced in the brain from a protein called Tyrosine which is absorbed into brain cells from the blood. L-dopa is also the basis of many treatments for Parkinson’s (such as Levodopa – commercial versions of Levodopa include ‘Sinemet’).
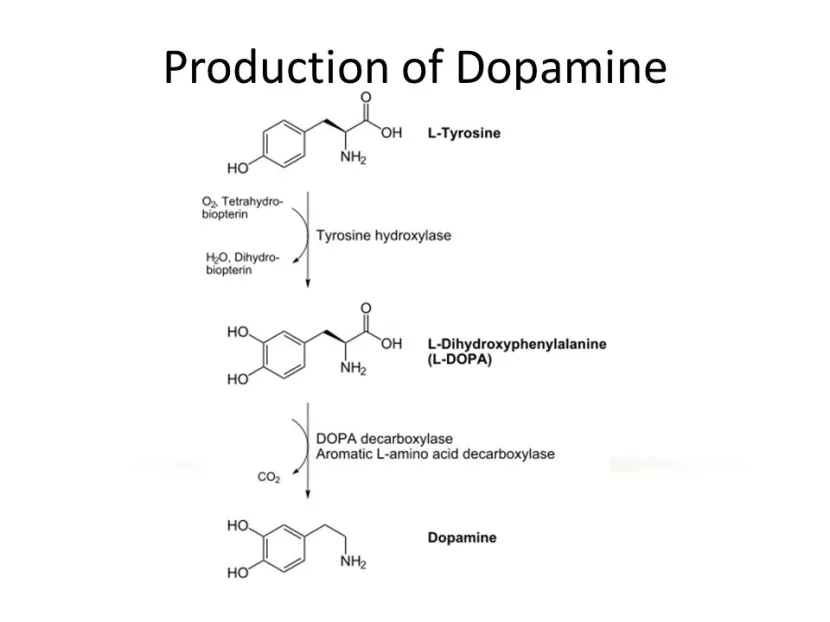
The production of dopamine. Source: Slideplayer
So AADC helps to produce dopamine, but why is dopamine important?
Dopamine is a chemical in the brain that helps us to move freely. Without it, our movements become inhibited – as in the case of Parkinson’s.
The majority of the dopamine produced in your brain is made in a region called the substantia nigra, deep inside you brain. These dopamine producing cells also generate another chemical called neuromelanin, which has a dark colouration to it – making it visible to the naked eye. As you can see in the image below, the substantia nigra region is easy to see on the section of healthy control brain on the left, but less visible on the section of brain from a person who passed away with Parkinson’s.

The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinson’s disease brain (right). Source:Memorangapp
The dopamine neurons of the substantia nigra release their dopamine in different areas of the brain. The primary regions of that release are areas of the brain called the putamen and the Caudate nucleus. The dopamine neurons of the substantia nigra have long branches/projections (called axons) that extend a across the brain to the putamen and caudate nucleus, so that dopamine can be released there.
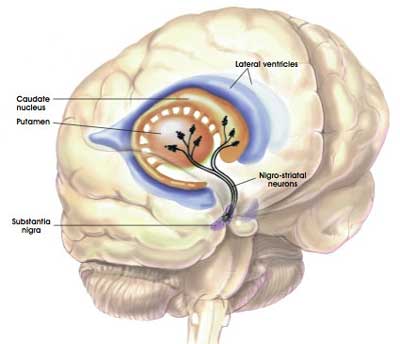
The projections of the substantia nigra dopamine neurons. Source: MyBrainNotes
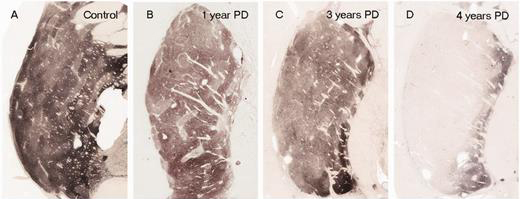
In Parkinson’s, these ‘axon’ extensions that project to the putamen and caudate nucleus gradually disappear as the dopamine neurons of the substantia nigra are lost. When one looks at brain sections of the putamen after the axons have been labelled with a dark staining technique, this reduction in axons is very apparent over time, especially when compared to a healthy control brain.
The putamen in Parkinson’s (across time). Source: Brain
So how does the VY-AADC01 virus help?
By injecting VY-AADC01 into the putamen of people with advanced Parkinson’s, Voyager is trying to make cells in that region (the putamen) start to produce AADC (which they usually don’t) and this will allow (in the use of Levodopa treatment) for the production of dopamine in the location where it is normally released by the (now absent) dopamine neurons. And this will hopefully help alleviate the motor features of the condition.
It must be understood, however, that the approach that Voyager Therapeutics is trialling here will not cure Parkinson’s, but it may make life a lot easier for those affected by it.
So what does the update from Voyager Therapeutics say?
The report expands on previous data from Voyager Therapeutics phase Ib clinical trial, in which 15 participants with advanced Parkinson’s have been treated with one of three doses of VY-AADC01. The participants were all treated with a single administration of VY-AADC01 (this is a surgical procedure which involves a syringe being inserted into the brain, and guided down into the putamen where the virus is injected).
Targeting the putamen (pink arrow indicates the syringe tract). Source: LCT
The primary endpoint (the main measure of assessment) of this Phase I study is the safety and tolerability of VY-AADC (as well as testing ascending doses of the virus), which is measured by the number of adverse events. The secondary endpoint of the study was a brain imaging approach (18F-DOPA) which allows for measures of the conversion of L-dopa into dopamine. Unfortunately, the update provides no information regarding the secondary endpoints.
The study is made up of three groups (or cohorts) of five patients, with each group receiving a different dose:
- The participants enrolled in Cohort #1 received a lower volume of the virus (up to 450 µL into the putamen on both sides of the brain) and a lower concentration of the virus (up to 7.5×1011 vector genome (vg)). These individuals were the first to be injected and they have been monitored/assessed the longest (36 months).
- The subjects in Cohort #2 received the medium volume dose of up to 1.5 × 1012 vg and up to 900 µL per putamen. These participants have been followed for 18 months.
- Participants enrolled in Cohort #3 received similar volumes compared to Cohort #2 (up to 900 µL per putamen), but three-fold higher concentrations (up to 4.5×1012 vg).
The participants enrolled in the study were:
- On average, 58 years of age with a Parkinson’s diagnosis for an average of 10 years.
- They were all candidates for surgical intervention (such as deep-brain stimulation) due to their disabling motor complications, despite treatment with optimal anti-Parkinsonian medication.
- At the start of the study (or baseline), the average ON-time without dyskinesia was 10.5 hours, and an average OFF-time of 4.6 hours. It should be noted though that participants in Cohort #3 started the trial with approximately 50% more dyskinesias at baseline than patients in Cohorts #1 and #2 (Unified Dyskinesia Rating Scale of 30.2 for Cohort #3 compared with a mean score of 19.2 and 17.4 for Cohorts #1 and #2, respectively).
- The average amount of Parkinson’s medications at baseline was 1,526 mg of oral levodopa equivalents per day.
During the study, the participants were instructed to reduce their daily doses of L-dopa (and related medications), resulting in reductions of 15%, 33% and 42% for Cohorts #1, #2 and #3, respectively, at six months post treatment with VY-AADC. As you can see in the figure below, these reductions in medication were sustained for Cohorts #1 until 18 months, at which point those individuals who received the lowest dose of VY-AADC began to require more L-dopa medication:
Reductions in PD medication. Source: Globenewswire
A single treatment of VY-AADC resulted in a durable and clinically meaningful 3.5-hour improvement in ON-time without troublesome dyskinesias over baseline measurements at 18 months in Cohort #2. This measure was based on participant assessed personal dairy measures:
Increases in ON-time without troublesome dyskinesias. Source: Globenewswire
Voyager Therapeutics believes that the reduced improvement of 1.5 hours (at 12 months) in Cohort #3 may have resulted from this group lowering their L-dopa treatment by a greater extent compared to participants in Cohorts #1 and #2. This is something that the company would like to explore in follow up trials.
In addition to measures of motor function, the researchers also found that VY-AADC01 improved patients’ quality of life (as measured by the Unified Parkinson’s Disease Rating Scale (UPDRS) and the participant-reported 39-item Parkinson’s Disease Questionnaire (PDQ-39)). For the PDQ-39, VY-AADC01 improved (reduced) patients’ score by a mean change of -8.4 and -9.1 for Cohorts 2 and 3, respectively (over baseline at 12 months):
Improvements in PDQ-39 scores. Source: Globenewswire
Wow! This is fantastic… right?
Yes, the results are very pleasing (especially given the failures of several previous gene therapy approaches for Parkinson’s, such as the AAV-neurturin trial – Click here to read more about this).
But there are a couple of aspects of the study to keep in mind when considering the results:
- It is an open label study, meaning that the participants know that they are in the treatment group (there was no blinding). What the researchers need to do now is conduct a phase II randomised trial that includes a control group. This is difficult in advanced Parkinson’s as it is asking people to partake in a study that may have no treatment benefit for them (if they are assigned to the control group).
- The company did not provide any information regarding the brain imaging, which means that we have no way of knowing how effective the virus is inside the brain – how much of an increase in dopamine production was caused by VY-AADC01? How much of a biological effect is the treatment actually having?
Interesting. Is anyone else working on gene therapy for Parkinson’s?
Yes, and a good example is Voyager Therapeutic’s competition: Oxford BioMedica.

Source: Oxford BioMedica
The scientists at Oxford Biomedica are using their virus (a product called ‘Prosavin’ (or OXB-101)) to transfer three genes – aromatic amino acid dopa decarboxylase (AADC – the same as Voyager Therapeutics (above)), tyrosine hydroxylase (TH), and GTP-cyclohydrolase 1 (GCH1) – into cells in the Putamen. These three genes are all involved in the production of the chemical dopamine.

GCH1, TH and AADC in dopamine production. Source: ScienceMag
The scientists at Oxford Biomedica are hoping to reprogram cells in the putamen to start making dopamine, and that this reprogramming would result in increased levels of dopamine in the brain and reduce the need for L-dopa treatment.
Preclinical research in rodent and primate models of Parkinson’s indicated very positive results using the Prosavin virus (Click here for more information on this), and the company moved towards testing the product in a clinical trial. The trial was completed in April 2012, and the results of the study were published in 2014:

Title: Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy forParkinson’s disease: a dose escalation, open-label, phase 1/2 trial.
Authors: Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, Kelleher M, Deeley S, Iwamuro H, Lefaucheur JP, Thiriez C, Fenelon G, Lucas C, Brugières P, Gabriel I, Abhay K, Drouot X, Tani N, Kas A, Ghaleh B, Le Corvoisier P, Dolphin P, Breen DP, Mason S, Guzman NV, Mazarakis ND, Radcliffe PA, Harrop R, Kingsman SM, Rascol O, Naylor S, Barker RA, Hantraye P, Remy P, Cesaro P, Mitrophanous KA.
Journal: Lancet. 2014 Mar 29;383(9923):1138-46.
PMID: 24412048
The clinical study was an open-label trial conducted over 12 months at two research centres (France and UK). It was designed to assess the safety and efficacy of ProSavin after the virus was injected into the striatum on both sides of the brain in 15 people with advanced Parkinson’s. Both research centres were registered as separate trials at ClinicalTrials.gov (NCT00627588and NCT01856439).
ProSavin was found to be safe and well tolerated in the study participants. No serious adverse effects related to the virus (or surgical procedure) were reported, and significant improvements in the motor issues associated with Parkinson’s were found (the average UPDRS score off medication in all of the patients at 12 months had improved from 38 at the start of the study to 27 (p=0·0001).
This video provides an explanation of the first clinical trial of Prosavin:
Following the completion of the clinical study, Oxford Biomedica decided to move ahead with a more potent virus, known as OXB-102. Preclinical testing indicates that OXB-102 is at least five-fold more potent than ProSavin (based on behavioural and movement analysis). Oxford BioMedica is currently working on the regulatory approval for a planned three cohort phase I/II study of OXB-102 in Parkinson’s which will be conducted (in the UK and France). That trial will begin this year (2018).
What does it all mean?
Gene therapy is the future of medicine for many diseases.
Using DNA to treat medical conditions rather than pills represents an attractive approach to treating various conditions. While pills – once consumed – have a systemic effect (across the whole body, resulting in ‘off-target’ side effects), gene therapy can be specifically targeted to a region of interest (either by surgical delivery or possibly by carefully engineered viruses that allow for non-invasive gene delivery – click here to read a previous post on this topic).
I am pleased by the progress of the Voyager therapeutics trials, and will keep following them with interest.
But it is important for readers to remember that this is not a curative treatment.
The VY-AADC01 product will only allow the brain to produce more dopamine and alleviate the motor features of Parkinson’s. While this treatment may allow individuals to reduce the number of tablets they need to take each day, it will not slow the progression of Parkinson’s. Having said that, VY-AADC01 may provide a significant improvement in quality of life to those with more advanced Parkinson’s, and in the absence of a curative treatment, an addition treatment options is better than nothing.
I am now looking forward to seeing the results of a phase II or III trial for VY-AADC01.
EDITOR’S NOTE: Voyager Therapeutics and Oxford BioMedica are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. Voyager Therapeutics and Oxford BioMedica have not requested that this material be produced, nor has the author had any contact with these companies or any associated parties. This post has been produced for educational purposes only.






Thank you again Simon
Sent from my iPhone
>
LikeLike
You’re welcome Scott!
Sent from my rubbish laptop
LikeLike
Keep up the great work, Simon!!! Always makes me look forward to the next informative article!!! You are the best!!
LikeLike
Thanks Dave – glad you like the material.
Kind regards,
Simon
LikeLike
Simon,
Thank you for the very informative update. I have three questions:
1. Should we see similar benefit from “pharmaceutical” methods that increase AADC production? My understanding is that vitamin B6 could be used for this.
2. It is my understanding that AADC affects production not only of dopamine but of other neurotransmitters as well. How significant is this?
3. What would be the role of tyrosine kinase in the process illustrated in ScienceMag above?
LikeLike
Hi Felix,
Thanks for your interesting questions:
1. AADC is already quite widely present inside (and outside) the brain. Lots of different cell type produce it because (as you say) AADC is involved in the production of many different neurotransmitters/modulators. And this can result in complications as levels of L-dopa medication increase over time in Parkinson’s. Given this wide spread presence, I am not sure that pharmaceutically enhancing AADC is a pathway forward, and this is also why the Voyager researchers are trying to localise the viral-induced production of AADC to the putamen – the region where the bulk of the dopamine in our brain is released. I am not aware of vitamin B6 actually increasing levels of AADC – I would be interested to learn more about this. I do know that AADC requires vitamin B6 to convert L-dopa into dopamine (and 5-hydroxytryptophan into serotonin).
2. Yes, AADC is not only involved in the production of dopamine, but a bunch of different neuromodulators/transmitters (including serotonin). So there is the possibility of increases in other things at the same time as dopamine. Voyager are basically betting on enough L-dopa being present via the medication to swing things in favour of dopamine. The idea behind the trial is nice – localised production of dopamine, allowing for the reduction of L-dopa treatment – but one has to ask if the cells now producing the dopamine can handle the production of dopamine? Can those cells package the dopamine and release it in an organised fashion while still doing the other stuff they are supposed to do? The results in this update are very positive, but a lot of information is missing unfortunately (some of which the company itself probably doesn’t have yet). It would be interesting though to see things like dopamine and serotonin brain imaging data. Maybe in the next update. We shall see.
3. Tyrosine Hydroxylase (TH) is not actually a kinase, but rather it is a rate-limiting enzyme in the production of dopamine. It converts tyrosine (coming in from the blood) into L-dopa via hydroxylation (or the introduction of a hydroxyl group (-OH) – a good review of this process is: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3065393/)
Many thanks for your interesting questions.
Kind regards,
Simon
LikeLike
Simon,
Thank you for the detailed answers. Here is the link to the article on treatment of AADC deficiency that has explanation of vitamin B6 role in AADC synthesis:
https://ojrd.biomedcentral.com/articles/10.1186/s13023-016-0522-z#Sec33
I hope it helps.
Felix
LikeLike
Don’t know about how accurate this article is (see link below) – but seems like disappointing news for Voyager effort:
https://seekingalpha.com/article/4184952-voyagers-parkinsons-gene-therapy-likely-fail
LikeLike
Hi KJJ,
Thanks for the comment. I also saw this article and I am extremely weary about leaving the link here in the comments section of this website. I am also VERY reluctant to comment on it. While our agenda here at the SoPD is education, the website you are linking to is – it is fair to say – all about financial speculation.
I will say this: At face value, the linked article provides a somewhat negative outlook for the Voyager clinical trial. But the comments section of the article actually makes for interesting reading. There, the author admits that he does not have a biology/medical background and regarding the trial, he admits that he is “not 100% certain it will fail”. He has also had to correct the article based on reader comments, and when pressed by readers about the biology underlying the treatment or certain aspect about the design of the trial, the author’s answers are not exactly reassuring. The author’s thesis also relies on the question of why Sanofi walked away from exercising its ex-US options (that is owning the treatment everywhere except the US). Such a move can easily be interpreted as a negative for the treatment, but there could be many different reasons for Sanofi to choose to play like that.
Not much can really be determined at Phase I (other than safety and dosing). Thus, we will wait to see the results of the phase II/III results before passing judgement on the treatment.
Kind regards,
Simon
LikeLike