|
The results of a small clinical study evaluating the safety and tolerability of Ursodeoxycholic acid (or UDCA) in people with Parkinson’s have recently been published. UDCA is a naturally occurring bile acid that is used in the treatment of gallstones. More recently, however, researchers have reported that this clinically available medication has beneficial effects in models of Parkinson’s. The clinical study that has recently been published suggests that UDCA is safe and well tolerated in people with Parkinson’s, and warrants further investigation in larger clinical trials. In today’s post, we will discuss what UDCA is, we will consider some of the previous research in models of Parkinson’s, we will review the results of the clinical trial, and then we will discuss what may happen next.
|
 Source: Youtube
Source: Youtube
How often do you consider your gallbladder?
Excuse me?
Your gallbladder. How often do you think about it?
Uhh,….never?
And I would believe that. It is one of the less appreciated organs. A pear-shaped, hollow organ located just under your liver and on the right side of your body. Its primary function is to store and concentrate your bile. What is bile you ask? Bile is a yellow-brown digestive enzyme – made and released by the liver – which helps with the digestion of fats in your small intestine (the duodenum).
 Source: Mayoclinic
Source: Mayoclinic
One of the down sides of having a gall bladder: gallstones.
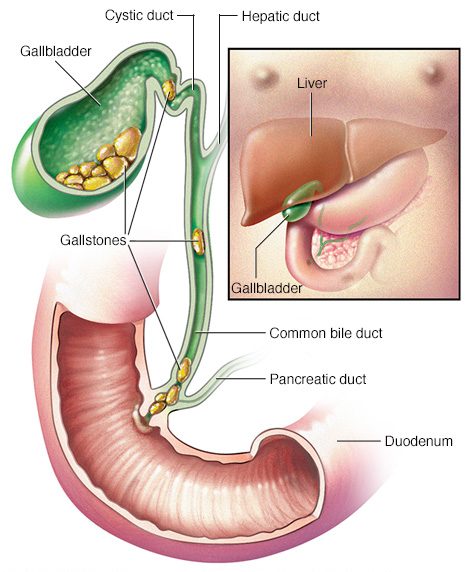
Gallstones are hardened deposits that can form in your gallbladder. About 80% of your average gallstone is cholesterol. The remaining 20% of a gallstone is made of calcium salts and bilirubin. Bilirubin is the yellow pigment in bile. When the body produces too much Bilirubin or cholesterol, gallstones can develop.
 Gallstones – ouch! Source: Healthline
Gallstones – ouch! Source: Healthline
About 10-20% of the population have gallstones (Source), but the vast majority experience no symptoms and need no treatment.
Interesting intro, but what does any of this have to do with Parkinson’s?
One of the treatments for gallstones is called UDCA. And recently we learned the results of a clinical trial in which UDCA is being “repurposing” as a treatment for Parkinson’s.
What is UDCA?
UDCA = Ursodeoxycholic acid (also known as ursodiol).
Ursodeoxycholic acid/UDCA is a bile acid. It is a naturally occurring chemical that changes the composition of bile and helps to dissolve gallstones.
UDCA was first discovered in bears, hence the use of the root-word for ‘bear’ (urso-) in its name.
 Cute, but don’t get too close. Source: Constantinealexander
Cute, but don’t get too close. Source: Constantinealexander
How does a a bile acid help with Parkinson’s?
Back in 1994, some researchers reported something very interesting about UDCA:
 Title: Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group.
Title: Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group.
Authors: Poupon RE, Poupon R, Balkau B
Journal: N Engl J Med. 1994 May 12;330(19):1342-7.
PMID: 8152446 (This article is OPEN ACCESS if you would like to read it)
In this study the researchers were looking for a new therapy for primary biliary cirrhosis.
Primary biliary cirrhosis is a liver disease. It is an autoimmune condition (meaning that your immune system starts to attack your body) which causes a slow, progressive destruction of the bile ducts of the liver, resulting in the build up of bile and other toxins in the liver.
Given that ursodiol (UDCA) has is not toxic to liver cells in humans, the researcher hypothesised that long-term treatment with this drug might displace the build up of bile acids and thus reduce their toxicity in primary biliary cirrhosis.
Crazier ideas have worked I suppose.
But guess what: It actually did work!
The investigators recruited and randomly assigned 145 patients with biopsy-proved primary biliary cirrhosis to receive either ursodiol (72 patients) or a placebo (73 patients) for 2 years. They found that long-term ursodiol therapy slowed the progression of the condition and reduced the need for liver transplantation.
Interesting, but again, what does this have to do with Parkinson’s?
Well, additional studies found that this ‘rescue effect’ may actually be due to cellular protective mechanisms rather than simply displacing the build up of other bile acids.
These studies included this one:
 Title: A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation
Title: A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation
Authors: Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ
Journal: J Clin Invest. 1998 Jun 15;101(12):2790-9.
PMID: 9637713 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers found that UDCA treatment in both hepatocytes and non-liver cells could block apoptosis (or programmed cell death). Apoptosis occurs when a cell is sick or damaged and it decides to shut down and die, initiating a programme of self destruction.
The investigators exposed liver cells to a range of apoptosis-inducing agents (from toxic doses of chemicals to apoptosis-causing proteins), and they found that co-administration of UDCA with each of these was associated with a 50-100% inhibition of apoptotic changes. They concluded that their “results suggest that UDCA plays a central role in modulating the apoptotic threshold” of the cells they tested.
|
RECAP #1: Ursodeoxycholic acid (UDCA) is a naturally occurring bile acid that is medically used to help breakdown gallstones. Recently researchers have reported that UDCA has beneficial properties outside of this primary function.
|
I’m still waiting for the connection to Parkinson’s. Are we getting close?
We have arrived.
Subsequent to these previous studies, research groups have observed similar beneficial effects with UDCA in models of Parkinson’s:

Title: Ursodeoxycholic acid suppresses mitochondria-dependent programmed cell death induced by sodium nitroprusside in SH-SY5Y cells.
Authors: Chun HS, Low WC.
Journal: Toxicology. 2012 Feb 26;292(2-3):105-12.
PMID: 22178905
In this study, the researchers demonstrated that UDCA could protect dopamine cells grown in cell culture from apoptosis, and they also found that UDCA is doing this by regulating specific cell survival pathways (PI3K-Akt/PKB).
And the neuroprotective effects of UDCA in models of Parkinson’s have also been independently found in a large screening study conducted by researchers at the University of Sheffield (UK):

Title: Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease
Authors: Mortiboys H, Aasly J, Bandmann O.
Journal: Brain. 2013 Oct;136(Pt 10):3038-50.
PMID: 24000005
In this study, the investigators took 2000 drugs (including 1040 licensed drugs and 580 naturally occurring compounds) and conducted a massive screen to identify drugs that could rescue mitochondrial dysfunction.
What is mitochondrial dysfunction?
Mitochondria are the power house of each cell. They provide the cell with energy, which helps to keep the lights on. Without them, the lights go out and the cell dies.

Mitochondria and their location in the cell. Source: NCBI
In certain genetic forms of Parkinson’s, the mitochondria in cells becomes dysfunctional and may not be disposed of properly. One of those genetic forms of Parkinson’s involves genetic errors (or variations) in a section of DNA (or gene) called PARK2 (also known as PARKIN – click here to read a previous SoPD post related to this). In cells with PARK2 genetic variants, mitochondria become stressed and are not disposed of correctly.
In their huge screen of 2000 drugs, the researchers in Sheffield used skin cells from people with PARK2 genetic mutations for their study. They identified 15 drugs that could rescue the mitochondria dysfunction in the PARK2 skins cells. Of those 15 compounds, two were chosen for further functional analysis. They were:
- Ursocholanic acid
- Dehydro(11,12)ursolic acid lactone
(Did anyone else notice that both of these compounds have the root word for ‘bear’ in them? Coincidence? I think not)
Neither ursocholanic acid nor dehydro(11,12)ursolic acid lactone are FDA-licensed drugs. In fact, we have little if any information regarding their use in humans. Given this situation, the researchers turned their attention to a chemically related compound: UDCA.
 UDCA. Source: Wikipedia
UDCA. Source: Wikipedia
When the researchers tested UDCA on PARK2 skin cells, and they found that the drug rescued the mitochondrial function in those cells.
They next tested UDCA on skin cells from people with Parkinson’s who had mutations in another Parkinson’s-associated gene, called PARK8 (or LRRK2) gene (G2019S; Click here for a previous SoPD post on this topic). The researchers had previously found impaired mitochondrial function and morphological issues in skin cells taken from people with PARK8-associated Parkinson’s (Click here to read more about this), and other groups had reported similar findings (Click here for more on this).
And when they treated the PARK8 cells with UDCA, guess what happened?
UDCA was able to rescue the mitochondrial effect in those cells as well!
And very recently, the researchers in Sheffield have demonstrated that UDCA can also correct mitochondrial dysfunction in skin cells for people with Alzheimer’s-associated genetic variants (Click here to read more about this).
Obviously, these results excited the Sheffield scientists and they set up a collaboration with researchers at York University (UK) and from Trondheim (Norway), to look at the potential of UDCA in rescuing the fate of PARK8 flies. The results of that study were published in this report:

Title: UDCA exerts beneficial effect on mitochondrial dysfunction in Lrrk2 (G2019S) carriers and in vivo.
Authors: Mortiboys H, Furmston R, Bronstad G, Aasly J, Elliott C, Bandmann O.
Journal: Neurology. 2015 Sep 8;85(10):846-52.
PMID: 26253449 (This article is OPEN ACCESS if you would like to read it).
The researchers tested UDCA on flies (or drosophila) with specific PARK8/LRRK2 mutations (G2019S) display a progressive loss of photoreceptor cell function in their eyes. The mitochondria in the photoreceptor cells are swollen and disorganised. When the investigators treated the flies with UDCA, they found approximately 70% rescue of the photoreceptor cells function.
The researchers in Sheffield concluded that UDCA has a marked rescue effect on cells from a Parkinson’s-associated gene mutation model, and they proposed that “mitochondrial rescue agents may be a promising novel strategy for disease-modifying therapy in LRRK2-related PD, either given alone or in combination with LRRK2 kinase inhibitors” (Click here to read a previous post on the topic of LRRK2 inhibitors).
And this study was followed by another more recent report (from a different research group) which involved testing UDCA in rodent models of Parkinson’s:

Title: Ursodeoxycholic Acid Ameliorates Apoptotic Cascade in the Rotenone Model of Parkinson’s Disease: Modulation of Mitochondrial Perturbations.
Authors: Abdelkader NF, Safar MM, Salem HA.
Title: Mol Neurobiol. 2016 Mar;53(2):810-7.
PMID: 25502462
These researchers found UDCA rescued a rodent model of Parkinson’s (involving the neurotoxin rotenone). UDCA not only improved mitochondrial performance in the rats, but also demonstrated anti-inflammatory and anti-cell death properties.
Given all this research, the Parkinson’s research community have been very keen to test UDCA in clinical trials for Parkinson’s.
|
RECAP #2: Researchers assessing cellular function in skin cells collected from people with Parkinson’s found that UDCA exhibits beneficial properties. These effects have been further explored in additional models of Parkinson’s with similar results.
|
Has anyone testing UDCA in the clinic for Parkinson’s?
Yes.
And this week we learned the results of one clinical study investigating UDCA in Parkinson’s.
Over the last few years, there has been a small Phase I clinical trial of UDCA in individuals with Parkinson’s being conducted at the University of Minnesota – Clinical and Translational Science Institute.
This week the results of that study were published:
 Title: Pharmacokinetics, Safety, and Tolerability of Orally Administered Ursodeoxycholic Acid in Patients With Parkinson’s Disease-A Pilot Study.
Title: Pharmacokinetics, Safety, and Tolerability of Orally Administered Ursodeoxycholic Acid in Patients With Parkinson’s Disease-A Pilot Study.
Authors: Sathe AG, Tuite P, Chen C, Ma Y, Chen W, Cloyd J, Low WC, Steer CJ, Lee BY, Zhu XH, Coles LD.
Journal: J Clin Pharmacol. 2020 Feb 12. [Epub ahead of print]
PMID: 32052462
In this study, the researchers recruited 5 people with Parkinson’s and they conducted an open-label, multiple-ascending-dose study of oral UDCA (Click here to read more about this).
What does that mean?
‘Open-label’ refers to the fact that everyone involved in the study (participants and investigators) knew that they were receiving the treatment. This can introduce different levels of bias (such as investigator bias or placebo response in the participants), but given that this was the first time UDCA was being tested in Parkinson’s, safety was the primary consideration. ‘Multiple-ascending-dose’ refers to the participants being administered more than one dose, and those doses will be increasing as the study continues. And ‘oral UDCA’ refers to the fact that UDCA is a treatment taken orally.
The researchers also wanted to assess whether UDCA will increase levels of ATP in the brains of individuals with Parkinson’s.
What is ATP?
ATP (or Adenosine Triphosphate) is the fuel which cells run on.
The mitochondria we were discussing up above are very efficient at converting nutrients from food into ATP. And we need HUGE amounts of ATP in order to do everything we do at any moment of the day.
FUN FACT: The average human body produces/recycles its own weight in ATP every day!
In the Minnesota UDCA study, the participants were administered daily UDCA treatment for six weeks. And the dose that they were receiving gradually increased over time (from 15 mg/kg/day in week 1, to 50 mg/kg/day from week 3 to week 6).
The researchers found that these doses were safe and generally well tolerated. The treatment also resulted in modest increases in ATP levels (based on brain imaging techniques).
Clinical assessments also suggested possible improvements in Parkinson’s symptoms/features (using the Unified Parkinson’s Disease Rating Scale (UPDRS parts I-IV), but the investigators were quick to point out in their report that the study was small and open label, which could lead to “placebo responses”. So the clinical results must be taken with a grain of salt. But the researchers suggested that based on the results of the study “a larger double-blind clinical trial is now warranted“.
|
RECAP #3: The Minnesota UDCA study found that daily treatment with UDCA was safe and generally well tolerated in people with Parkinson’s. Brain imaging studies and clinical evaluations suggested additional benefits, but given the open label nature of the study, a larger and longer clinical trial is now required.
|
So how long do we have to wait for the next study to start?
We don’t have to wait at all.
Another UDCA study has already started.
The trial, known as the “UP Study” (“UDCA in Parkinson’s”) is being conducted at two research centres in the UK – one in Sheffield and one in London. The main investigational team is in Sheffield – led by Prof Oliver Bandmann – and they are working in collaboration with Prof Tom Foltynie and his team at University College London Hospitals.
 Prof Bandmann & Dr Mortiboys – Sheffield University. Source: Medicine.dept.shef
Prof Bandmann & Dr Mortiboys – Sheffield University. Source: Medicine.dept.shef
The study – involving 30 participants who are less than 3 years since diagnosis – is a Phase II, placebo controlled, double blind, randomised clinical trial assessing the longer-term safety and tolerability of 30 mg/kg daily dosing of UDCA in people with Parkinson’s.
The participants have been allocated randomly (in a 2:1 ratio) to receive either UDCA or a placebo, for a total of 48 weeks (Click here to read more about the details of the study).
The primary outcome measures of the study are the safety and tolerability of UDCA in Parkinson’s at a dose of 30 mg/kg (which is within the dose range tested by the Minnesota UDCA study) over a long period of time. Additional clinical and biological endpoints are also being explored. The results of this study should be available next year (2021).
Interesting. So what does it all mean?
Not just yet.
There was another (related) bit of UDCA research this week. Prof Bandmann and the Sheffield team (in collaboration with researchers in Oxford) published this cell culture-based study:
 Title: Deep phenotyping of peripheral tissue facilitates mechanistic disease stratification in sporadic Parkinson’s disease.
Title: Deep phenotyping of peripheral tissue facilitates mechanistic disease stratification in sporadic Parkinson’s disease.
Authors: Carling PJ, Mortiboys H, Green C, Mihaylov S, Sandor C, Schwartzentruber A, Taylor R, Wei W, Hastings C, Wong S, Lo C, Evetts S, Clemmens H, Wyles M, Willcox S, Payne T, Hughes R, Ferraiuolo L, Webber C, Hide W, Wade-Martins R, Talbot K, Hu MT, Bandmann O.
Journal: Prog Neurobiol. 2020 Feb 11:101772. [Epub ahead of print]
PMID: 32058042
In this study, the researchers collected fibroblasts from 100 people with Parkinson’s and from 50 age-matched control people without Parkinson’s
What is a fibroblast?
Fibroblasts are cells that are found in connective tissues of the body, like the skin. They play important roles in the maintenance of structural integrity of these tissues, as well as injury repair.
 The location of fibroblasts in the skin. Source: PP
The location of fibroblasts in the skin. Source: PP
Fibroblasts are also very robust and easy to grow in cell culture, and as such they represent a useful and accessible means of personalising medicine. By taking a simple skin biopsy, researchers can grow cells in culture from a specific person with Parkinson’s and conduct various tests or administer particular treatments to explore the characteristics of that person’s Parkinson’s.
 Fibroblasts growing in culture. Source: Wikipedia
Fibroblasts growing in culture. Source: Wikipedia
The researchers in Sheffield & Oxford were interested in assessing whether they could use fibroblasts to better subtype people with Parkinson’s.
What do you mean by subtype?
If you go along to any Parkinson’s support group, you will see that there is a great deal of variability between people with Parkinson’s in terms of things like their symptoms as well as their speed of progression. So much variability that researchers now believe that we may be dealing with a number of different conditions that all have the same sort of appearance as “Parkinson’s disease”, but they could have different underlying biology and causes.
This belief has led to numerous research efforts that are trying to tease out the different types of “Parkinson’s”, which will hopefully lead to more focused treatments in future.
In their study, the Sheffield & Oxford researchers investigated two aspects of the fibroblast cells function:
- Energy production (mitochondrial health)
- Waste disposal (or lysosomal function – click here to read a previous SoPD post on this topic)
Curiously, the researchers found little difference between the Parkinson’s fibroblasts and those collected from control subjects in terms of these two aspects of cellular functioning. But they did note that there was a lot more variability within the Parkinson’s samples.
Important to our discussion in this post today, however, is that when the investigators treated some of the fibroblasts with UDCA, they observed that the treatment returned the Parkinson’s fibroblasts to a normal level of mitochondrial functioning – providing further support for the ongoing repurposing effort of this clinically available medication.
So what does it all mean?
Ursodeoxycholic acid (or UDCA) is a naturally occuring bile acid that is used in the treatment of gallstones. Recently, a number of research groups have demonstrated that this medication also has beneficial properties in models of Parkinson’s, which has led to the initiation of clinical trials to see if this treatment should be re-positioned for used in PD.
This week the results of a small safety/tolerability that was conducted in Minnesota have been published. The findings of the study suggest that UDCA is safe and generally well tolerated in people with Parkinson’s, and based on this (and some interesting brain imaging and clinical data) the researcher concluded that larger and longer clinical evaluations of this treatment are now required.
A Phase II clinical trial of UDCA in people with Parkinson’s is now ongoing and we will hopefully have the results of that study next year. And that will – fingers crossed – hopefully provide support for the further development of this medication as a potential Parkinson’s treatment.
Like I said, fingers crossed.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE – The author of this post is an employee of The Cure Parkinson’s Trust which is supporting the UP study (along with the Van Andel Institute, the JP Moulton Charitable Foundation, and the pharmaceutical company PRO.MED.CS). Neither the UP study team nor CPT has not asked for this post to be written. This post has been provided by the author solely for the purpose of sharing what the author considers very interesting information.
The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by a research scientist, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. Please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Stateforesters


What are the implications for someone who has had a gallbladder removed and was subsequently diagnosed with Parkinson’s disease?
LikeLiked by 1 person
Thank you Simon! I have two questions: 1. couldn’t it be that this Ursodeoxycholic acid would help restore correct enzymatic function in the stomach (by making the latter more acid) and so help break down the necessary nutriments for the brain/body (in addition to directly restoring ATP production as attested through the skin culture experiments). 2. I’ve read here and there that the use of benzodiazepines was detrimental to correct mitochondrial function. Do you know of solid evidence pointing towards this fact?
LikeLike
All this testing… just give me the stuff and be done with it! While I’m greatly encouraged by all the research and testing, there is still nothing more effective (for me, at least) than Sinemet 4x a day. DBS is on the radar, but is only a faint blip. When will something actually be available to the American public?
LikeLike