|
# # # # For a some time, issues with the gastrointestinal system has been associated with Parkinson’s. For example, gut-related complaints – such as constipation and irritable bowel syndrome – are believed to be risk factors for developing the condition. Researchers have recently been exploring the bacteria that inhabit the gastrointestinal system in the hope of identifying species of microbes that could be directly influencing the condition. Exactly how these tiny organisms might be doing that, however, remains a mystery. Now researchers have focused their attention on a particular type of protein that is being produced by some of those bacteria. It is called curli. In today’s post, we will discuss what curli is, explore what functions it has, and do a deep dive into some of the data suggesting it could be involved with Parkinson’s. # # # # |
 Me. In a skirt. Eating dirt. Not my worst moment. Source: Drqaisrani
Me. In a skirt. Eating dirt. Not my worst moment. Source: Drqaisrani
It has to be said that our gastrointestinal systems are incredibly robust.
When I think of all the rubbish I put down my throat as a toddler (and then of all the rubbish I put into my gut as an adult) I am bewildered as to how that 30 feet of digestive machinery is still functioning. And yet it does.
Reasonably well, at least. There is that whole ice cream thing, but let’s not dwell on that (Click here to learn more about that).
 Something’s missing in my life. Source: Morellisices
Something’s missing in my life. Source: Morellisices
Despite all the accolades for its robustness, our guts do represent one of the greatest opportunities for foreign organisms to invade our bodies. It is a very supportive, resource-rich environment for many microbes, and they can easily take up residence without us even knowing.
 Source: Huffington Post
Source: Huffington Post
And this is an important aspect of our guts, as it is becoming increasingly clear that some of these uninvited guests can have a very negative impact on our bodies.
Recently, there are been a huge amount of attention in Parkinson’s research focused on the gut and the bacteria that live within it for this very reason.
But what is the connection between the gut and Parkinson’s?
This is Prof Heiko Braak:
 Prof Heiko Braak. Source – Memim.com
Prof Heiko Braak. Source – Memim.com
Many years ago, Prof Braak – a German neuroanatomist – sat down and examined hundreds of postmortem brains from people with Parkinson’s.
He had collected brains from people at different stages of Parkinson’s and was looking for any kind of pattern that might explain where and how the condition starts. His research led to what is referred to as the Braak stages of Parkinson’s – a six step explanation of how the condition spreads up from the brain stem and into the rest of the brain (Click here to read more about this).
 The Braak stages of PD. Source: Nature
The Braak stages of PD. Source: Nature
Braak’s results also led him to propose that Parkinson’s may actually begin in the gut and then spread to the central nervous system (the brain). He based this on the observation that many brains that exhibited the very early stages of Parkinson’s had disease-related pathology in a population of neurons called the dorsal motor nucleus of the vagus nerve.
What is the dorsal motor nucleus of the vagus nerve?
The vagus nerve is the main connection between the gastrointestinal system and the brain.
It links the nerves surrounding the gut – collectively referred to as the enteric nervous system – with a region of the brain called the medulla oblongata, which sits in the brain stem at the top of the spinal cord.
 The vagus/vagal nerve connection with the enteric nervous system. Source: Nature
The vagus/vagal nerve connection with the enteric nervous system. Source: Nature
Braak and his colleagues found large deposits of the Parkinson’s-associated protein called alpha synuclein nerves surrounding the gut and the vagus nerve. These deposits were present even at very early stages of the condition, which supported his theory that maybe the disease was starting (or ‘originating’) in the gut.
This ‘gut to brain’ theory was supported by the fact that people with Parkinson’s often complaining of gastrointestinal problems (eg. constipation) and some of these issues have been suggested to predate the onset of other Parkinson’s symptoms.
Interesting, but what is alpha synuclein?
We talk about alpha synuclein a lot on this website – if you don’t want to read another description of this protein (I won’t be offended), just skip down to RECAP #1.
Alpha synuclein is one of the most common proteins in the brain (making up about 1% of the protein in neurons). The exact function of alpha synuclein is not well understood, but the current data suggests that it plays a role in multiple cellular functions – particularly in neurotransmitter release.
But in Parkinson’s, something changes.
For some reason, in many cases of Parkinson’s alpha synuclein protein starts to cluster and clump together. And this “aggregated” form of alpha synuclein appears to become toxic.
The aggregation of alpha synuclein is believed to lead to the appearance of Lewy bodies.
What are Lewy bodies?
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s. They are one of the classic hallmarks of Parkinson’s that a neuropathologist will look for in a postmortem brain in order to give a definitive diagnosis of PD (Click here for more on Lewy bodies).

A neuron, with the Lewy body within the cell. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells – see the image below where alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.
 Examples of Lewy neurites (stained in brown; indicated by arrows). Source: Wikimedia
Examples of Lewy neurites (stained in brown; indicated by arrows). Source: Wikimedia
And this build up of alpha synuclein is believed to be toxic for the affected cells, ultimately killing them.
How does alpha synuclein protein aggregate and become toxic?
When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure. Alone, it will look like this:
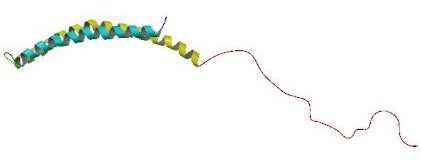 Alpha synuclein. Source: Wikipedia
Alpha synuclein. Source: Wikipedia
By itself, alpha synuclein is considered a monomer, or a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.
 Microscopic images of Monomers, oligomers and fibrils. Source: Brain
Microscopic images of Monomers, oligomers and fibrils. Source: Brain
It is believed that the oligomer and fibril forms of alpha synuclein protein gradually form what are referred to as amyloid proteins that ultimately give rise to Lewy bodies:

Parkinson’s associated alpha synuclein. Source: Nature
In this context, alpha synuclein is considered to be an amyloid protein.
|
# RECAP #1: Previous research of the gut suggest this organ may play an influential role in Parkinson’s. In addition to complaints like constipation, clustered (aggregated) form of alpha synuclein protein can be seen in some cases of PD, in both the gut and the nerve connecting the digestive system to the brain. There is evidence of Parkinson’s-associated pathology existing in the gut in some cases of PD before the neurodegeneration begins in the brain, supporting a ‘gut to brain’ theory of the condition. # |
What does ‘amyloid protein’ mean?
An amyloid protein is one which folds into a particular shape which allows many copies of that protein to stick together.
When a protein is first formed, it must be folded into their correct functioning shape in order for it to do its assigned task.
But if a protein remains only partially folded or completely misfolded, then it can acquire a shape which may encourage it to stick to other similarly shaped proteins, resulting in the production of ‘amyloid assemblies’. These assemblies are called amyloid fibrils.
 Source: Quora
Source: Quora
Alpha synuclein can form amyloid fibrils, and as a result, it is refered to as an amyloid protein.
It is important to understand that there are lots of different protein considered to be amyloid proteins – alpha synuclein is not unique here – and they are associated with a variety of medical conditions, including diabetes and Alzheimer’s (Click here for a partial list).
But how is misfolded alpha synuclein protein associated with the gut?
Well, this is where the story starts to get a rather interesting.
You see, some of the bacteria in our gut have the ability to form amyloid proteins themselves.
The most well studied of these bacterial amyloid proteins is called curli, which was first discovered in the late 1980s on Escherichia coli gut bacteria:
 Escherichia coli – cute, huh. Source: Vallhebron
Escherichia coli – cute, huh. Source: Vallhebron
Gut bacteria, like Escherichia coli and Salmonella, produce large amounts of curli protein which bind together to form amyloid fibres. But these amyloid fibres have very important functions. They are involved in the adhesion of these bacteria to surfaces within the gut, cell-cell contacts, and help to promote cell aggregation (“community behaviour”) ultimately aiding in host colonisation.
In this manner, curli fibers are involved in ‘biofilm formation’.
Biofilm formation?
Biofilm formation enables single-cell organisms to assume a multicellular lifestyle for a while. This “community behavior” facilitates survival in adverse environments like the gut (Click here to read more about Curli).
While this “community behavior” of curli demonstrates that amyloid proteins can have specific functions, they can also have undesirable consequences as well. For example, one problem with curli fibers is that they are potent inducers of inflammatory responses in the host.
What is an inflammatory response?
When cells in your body are stressed or sick, they begin to release tiny messenger proteins which inform the rest of your body that something is wrong.
When enough of these messenger proteins are released that the immune system becomes activated, it can cause an inflammatory response.
Inflammation is a critical part of the immune system’s response to trouble. It is the body’s way of communicating to the immune system that something is wrong and activating it so that it can help deal with the situation.
By releasing the messenger proteins (called cytokines), injured/sick cells kick off a process that results in multiple types of immune cells entering the troubled area of the body and undertaking very specific tasks.
 The inflammatory process. Source: Trainingcor
The inflammatory process. Source: Trainingcor
The strength of the immune response depends on the volume of the signal arising from those released messenger proteins. And curli fibers are very good at inducing these inflammatory responses.
But this is not the only thing curli fibers do.
A few years back, researchers have noticed something about curli that raised some eye brows.
They reported their finding in this study:
 Title: Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans.
Title: Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans.
Authors: Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, Roberts AM, Jagadapillai R, Liu R, Choe K, Shivakumar B, Son F, Jin S, Kerber R, Adame A, Masliah E, Friedland RP.
Journal: Sci Rep. 2016 Oct 6;6:34477.
PMID: 27708338 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to explore factors that may be responsible for the initiation of alpha synuclein protein aggregation. They were interested in curli because it had been previously reported that this protein had regions that were shared by alpha synuclein (Click here to read more about this).
To explore if curli could be helping to initiate the amyloid behaviour of alpha synuclein, the researchers used aged rats and genetically engineered C. elegans.
Rats I am familiar with, but what are C. elegans?
Caenorhabditis elegans (or simply C. elegans) are transparent nematode – also known as roundworms. They are about 1 mm in length, and they have very well characterised nervous systems (useless pub quiz fact: C. elegans have 302 neurons and 56 glial cells in total, which communicate through approximately 6400 chemical synapses, 900 gap junctions, and 1500 neuromuscular junctions – like I said, well characterised!).
 Caenorhabditis elegans – cute huh? Source: Nematode
Caenorhabditis elegans – cute huh? Source: Nematode
Given their well characterised nervous systems, C. elegans provide a useful tool for studying biology. They are easy to grow/maintain, they have an overall life span of 2-3 weeks, and researchers have developed a wide range of tools that allow for genetic manipulation to address specific questions.
So the researchers exposed both rats and C. elegans to E. coli bacteria that produced high levels of curli. The C. elegans had been genetically engineered to produce alpha synuclein protein (usually they don’t) and when they were fed curli-producing bacteria, the investigators observed an enhanced aggregation of alpha synuclein protein.
They also saw increased levels of alpha synuclein protein aggregation in the bowels of rats to E. coli bacteria that produced high levels of curli:
 Source: PMC
Source: PMC
These rats were administered the E coli bacteria once per week for 2-3 months. And note in the image above, that exposure to an E. coli bacteria that produced high levels of a mutant form of curli did not cause increased alpha synuclein protein aggregation (panels on the left in the image above). This suggests that the effect was not caused by the presence of the bacteria, but rather the curli protein itself.
Rats exposed to curli-producing E. coli bacteria also exhibited elevated alpha synuclein protein aggregation in the brain, as well as increased numbers of microglia (the resident immune cells in the brain) and raised levels of inflammation markers.
These results led the researchers to conclude that amyloid proteins being produced by bacteria in the microbiota may be “involved in the origination and maintenance of neurodegenerative disease”.
|
# # RECAP #2: Amyloid proteins can fold into a particular shape which allows many copies of that protein to stick together, forming amyloid fibrils. Alpha synuclein has been shown to have amyloid properties, but bacteria in the gut also produce amyloid proteins (for example Escherichia coli and Salmonella which produce the amyloid protein curli). Recently researchers have reported that curli produced by bacteria in the gut can enhance the aggregation of alpha synuclein and associated toxicity issues (neurodegeneration and behavioural complications). # # |
Has anyone ever replicated this research?
Yes, the results of that report have recently been independently replicated by other research groups. For example, this report published in February of this year:
 Title: A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice.
Title: A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice.
Authors: Sampson TR, Challis C, Jain N, Moiseyenko A, Ladinsky MS, Shastri GG, Thron T, Needham BD, Horvath I, Debelius JW, Janssen S, Knight R, Wittung-Stafshede P, Gradinaru V, Chapman M, Mazmanian SK.
Journal: Elife. 2020 Feb 11;9:e53111.
PMID: 32043464 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers used genetically engineered mice that produce high levels of human alpha synuclein, and they treated them with curli-producing E. coli bacteria. They found that curli promoted alpha synuclein pathology in both the gut and the brain. And this resulted in behavioral issues, including both intestinal and locomotor impairments.
Interestingly, the investigators also tested a purified subunit (or portion) of curli fibers, called CsgA. They found that CsgA alone was sufficient to accelerate alpha synuclein aggregation, both in cell culture and in mice.
But EVEN MORE interestingly, oral treatment of the amyloid inhibitor EGCG was able to reduce CsgA levels in the gut, limiting alpha synuclein aggregation in the brain, and lowering the behavioural issues.
What is EGCG?
Epigallocatechin Gallate (or EGCG) is a powerful catechin.

The chemical structure of EGCG. Source: GooglePatents
What is a catechin?
Catechins are a group of compounds that are found in tea, fruit, chocolate and wine. They belong to a family of nutrients called flavonoids (a large class of plant pigments), and have been linked to a variety of health benefits.
 Green tea – a liquid infusions of EGCG. Source: Dailymatcha
Green tea – a liquid infusions of EGCG. Source: Dailymatcha
Importantly, catechins (particularly EGCG) are potent antioxidants (Click here to read a previous SoPD post about EGCG).
EGCG is also remarkably good at blocking the production of alpha synuclein aggregates.
And there have been many studies that have demonstrated this effect:
 Title: EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers.
Title: EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers.
Authors: Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE
Journal: Nat Struct Mol Biol. 2008 Jun; 15(6):558-66.
PMID: 18511942
In this study, the researchers found that EGCG efficiently inhibits the formation of both alpha synuclein and Alzheimer’s associated beta amyloid fibrils. And it did this by directly binding to the native unfolded forms of the protein and prevented their conversion into the toxic forms of aggregates.
And other studies have reported even more impressive results:
 Title: EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity
Title: EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity
Authors: Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE.
Journal: Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):7710-5. doi: 10.1073/pnas.0910723107.
PMID: 20385841 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers found that EGCG has the ability to not only block the formation of of alpha synuclein fibrils and stabilize monomers of alpha synuclein, but it can also bind to alpha synuclein fibrils and restructure them into the safe/non-toxic forms of aggregates.
Has EGCG been tested in the clinic for Parkinson’s?
Yes, it has.
The efficacy and safety of EGCG was tested in 480 de novo (recently diagnosed) people with Parkinson’s in China in a study supported by the Michael J Fox foundation (Click here to learn more about the details of that trial). The study was started in 2007, and the participants were to be treated for 1 year with one of three different doses of EGCG or a placebo treatment. I am not sure if the results of this trial have ever been published (please correct me if I am wrong here), but the Michael J Fox Foundation have a short summary on their website which reads:
I am not sure if the results of this trial have ever been published (please correct me if I am wrong here), but the Michael J Fox Foundation have a short summary on their website which reads:
“To evaluate the safety, tolerability and efficacy of green tea polyphenols (GTPs) in slowing disease progression in patients with early PD, the team carried out a multi-center, double-blind, randomized, placebo-controlled, delayed-start study in 32 Chinese Parkinson Study Group (CPSG) sites. Study enrollees were 410 untreated PD patients with disease duration of less than five years and Hoehn & Yahr stage below 3 who were not heavy tea drinkers. Participants were randomized to one of three doses of GTP (0.4, 0.8 or 1.2 grams daily given in two equal oral doses) or matching placebo. After six months, placebo was switched to 1.2 grams daily of GTP. All patients were treated for 12 months.”
“The change in total UPDRS score from randomization to six months was significantly improved in GTP-treated groups as compared to the placebo group, but this was not observed at 12 months. In the delayed-start group, the change in UPDRS from six months (the start of active GTP) to 12 months was significantly improved. Insomnia was slightly increased in GTP-treated patients, but there were no other significant differences in adverse effects. GTP is well tolerated and appears to provide, at least, a mild symptomatic benefit in early untreated PD.” (Source)
There is also another clinical trial that completed last year, which was testing EGCG in individuals with multiple system atrophy (or MSA a condition very similar to Parkinson’s – Click here to read a previous SoPD post on MSA).
That more recent trial was called the PROMESA study, and the results have been published:
 Title: Safety and efficacy of epigallocatechin gallate in multiple system atrophy (PROMESA): a randomised, double-blind, placebo-controlled trial.
Title: Safety and efficacy of epigallocatechin gallate in multiple system atrophy (PROMESA): a randomised, double-blind, placebo-controlled trial.
Authors: Levin J, Maaß S, Schuberth M, Giese A, Oertel WH, Poewe W, Trenkwalder C, Wenning GK, Mansmann U, Südmeyer M, Eggert K, Mollenhauer B, Lipp A, Löhle M, Classen J, Münchau A, Kassubek J, Gandor F, Berg D, Egert-Schwender S, Eberhardt C, Paul F, Bötzel K, Ertl-Wagner B, Huppertz HJ, Ricard I, Höglinger GU; PROMESA Study Group.
Journal: Lancet Neurol. 2019 Aug;18(8):724-735.
PMID: 31278067
This study involved 92 individuals with MSA being randomly assigned to receive either EGCG (n=47) or placebo (n=45) for 40 weeks. The investigators found that the treatment did not modify disease progression in the treated individuals, but they did suggest that the study “was not powered to detect smaller changes.” (‘powered’ is a statistical term regarding how well a study will be able to detect a difference between groups; this study was powered to detect a 50% difference after 12 months).
There was a trend in the data suggest that the EGCG treated group performed better with regards to rate of progression, but this was not statistically significant. This may have been partly due to a potentially negative effect of the dose of EGCG used. The researchers found that 1200 mg of EGCG daily may be “toxic for an substantial proportion of patients with multiple system atrophy“.
It’s toxic???
The treatment used in the study was a capsule form of EGCG, but these have recently been called into question.
 The European Food Safety Authority (EFSA – the agency of the European Union that provides independent scientific advice and communicates on existing and emerging risks associated with the food chain) has recently warned that food supplement doses of EGCG at 800 mg or more per day may be associated with signs of liver damage.
The European Food Safety Authority (EFSA – the agency of the European Union that provides independent scientific advice and communicates on existing and emerging risks associated with the food chain) has recently warned that food supplement doses of EGCG at 800 mg or more per day may be associated with signs of liver damage.
The EFSA are quick to reassure that this is only related to food supplements. Liquid infusions of EGCG appear to be safe (Click here to read more about this).
So my take away from all of this is that EGCG has not yet really been thoroughly tested in Parkinson’s.
|
# # # RECAP #3: Independent research groups have replicated (and expanded on) the results suggesting that bacterially-produced curli increases alpha synuclein protein aggregation. They have also demonstrated that the alpha synuclein aggregation inhibitor – EGCG – can reduce the negative effect of curli in mice. The efficacy of EGCG in humans is yet to be thoroughly tested. # # # |
Interesting. What other effect could curli be having?
Very recently (like this week), this report was published:
 Title: In vivo synthesis of bacterial amyloid curli contributes to joint inflammation during S. Typhimurium infection.
Title: In vivo synthesis of bacterial amyloid curli contributes to joint inflammation during S. Typhimurium infection.
Authors: Miller AL, Pasternak JA, Medeiros NJ, Nicastro LK, Tursi SA, Hansen EG, Krochak R, Sokaribo AS, MacKenzie KD, Palmer MB, Herman DJ, Watson NL, Zhang Y, Wilson HL, Wilson RP, White AP, Tükel Ç.
Journal: PLoS Pathog. 2020 Jul 9;16(7):e1008591.
PMID: 32645118 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers demonstrated that curli (produced by bacteria in the gut) can cause an increase in inflammation. We discussed this above, but in this study, the investigators found that this increase in inflammation can result in an autoimmune response.
What is an autoimmune response?
An autoimmune response is an immune response within an organism against its own healthy cells and tissues. Any disease that results from such an immune response is called an autoimmune disease.

Different types of autoimmune diseases. Source: DrJockers
There has been evidence presented that Parkinson’s could be an autoimmune disease (Click here to read a previous SoPD post on the topic of autoimmunity), and it is interesting that the researchers of this new study found that curli-producing bacteria was associated with an increase in autoantibodies and joint inflammation in infected mice.
Despite focusing on joint inflammation, in a press release about the study the researchers behind the work actually noted that “The ability of bacterial curli to escape the intestine and reach the systemic circulation raises questions about a potential role in neurodegenerative processes.”
I look forward to learning more about any ongoing research that might be behind this statement.
So what does it all mean?
To have one by the short and curlies, refers to having complete control or dominance over someone. Specifically, the “short and curlies” refers to the hairs on the back of your neck (and not hairs from other parts of the body – which is the common misconception (source), so no apologies to those offended by the title of this post). If all the evidence is against you, it is said that the police (with their hand on the back of your neck) have you by the short and curlies.
The evidence supporting curli in the context of Parkinson’s is still evolving, but there are a few key considerations to keep in mind. For example, E coli is not a major component of the bacteria of our gut (or our microbiota). But there are several additional species that make amyloid proteins, including Streptococcus mutans, Staphlococcus aureus, Salmonella enterica, and Mycobacterium tuberculosis (Click here to read a good, short review on this topic). Therefore, investigations of the amyloid influence of the gut should not be solely focused on curli.
It will be interesting to see what impact therapeutics like Enterin Inc‘s ENT-01 (an alpha synuclein aggregation inhibitor that is only functional in the gut – click here to read an old SoPD post about this) may have on this process. But better methods of detecting and measuring alpha synuclein aggregation in the gut will be required in order for conclusive before and after measures of any therapeutic benefits. Enterin Inc is currently conducting a Phase IIa ‘KARMET’ clinical study of ENT-01 – which is scheduled to finish in October 2020, so hopefully we will learn more about this agent before the end of the year (Click here to read more about this study).
Phew, long post. I think that’s it for today.
Now it’s time to get back to putting rubbish in my gut.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITORIAL NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. Dietary changes can impact the effectiveness of treatment regimes. PLEASE speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Medicalxpress

The title of NCT03781791 is “Orally Administered ENT-01 for Parkinson’s Disease-Related Constipation (KARMET).”
However, from a recent paper by Michael Zasloff et al. (Enterin Inc et al.):
“Overproduction of αS in the enteric nervous system (ENS) and its chronic trafficking to the CNS may damage nerves and lead to Parkinson’s disease. Targeting the formation of αS aggregates in the ENS may therefore slow the progression of the disease.”
Gastrointestinal Immunity and Alpha-Synuclein (September 2019):
https://content.iospress.com/articles/journal-of-parkinsons-disease/jpd191702
Although (in KARMET) they are focusing on constipation, it seems they have hopes for much wider applicability?
LikeLike
I’ve just noticed that a related study record has very recently appeared on the Clinical Trials website (July 23, 2020).
NCT04483479 is titled “Phase 2b Follow-on Safety “Roll-over” Study (Rollover)”.
https://clinicaltrials.gov/ct2/show/NCT04483479?term=NCT04483479&draw=2&rank=1
Official Title: A Multicenter, Non-Randomized, Open-Label Study to Evaluate the Safety and Efficacy of Orally Administered ENT-01 in Improving Constipation and Neurologic Symptoms in Patients With Parkinson’s Disease and Constipation Over a 14-week Period.
Estimated Study Completion Date: December 31, 2021.
I hope they don’t decide to “roll-over” the publication of the results of the first trial!
LikeLike
Hi Simon,
thank you for this post. It combines several topics which I as a PwP am interested in. One of them is the green tea extract EGCG, which I have been taking for several years.
You are writing the following about the PROMESA study, that investigated the effect of EGCG on MSA patients:
“The investigators found that the treatment did not modify disease progression in the treated individuals, but they did suggest that based on the final numbers of participants completing the study, that it `was adequately powered to detect a disease-modifying treatment effect'”.
I am afraid that not every reader is familiar with the meaning of the word “power” in this statistical context. The full sentence from which you quote is as follows:
“These findings suggest that the study was adequately powered to detect a disease-modifying treatment effect of 50% on annual progression of UMSARS motor examination scores and that epigallocatechin gallate does not have this large effect.”
For me, this means that the authors could not show what they were hoping for and that they don’t blame an insufficient number of participants for this negative result. In many other studies, the authors admit that they could not achieve a statistically significant result, but instead of accepting their defeat, they rush to claim (without any valid quantitative reason) that with a larger sample size they would most likely have been able to show what they wanted to show. I prefer the way the authors of the PROMESA study handle this issue. Instead, they write that “the goal of reducing disease progression by 50% might have been too ambitious”.
Soon after the completion of the PROMESA study I had the opportunity to talk to one of the authors of the study. I asked him/her if he/she would take EGCG in my position as a person with young onset Parkinson’s in spite of the disappointing study results. He/she replied that one could do so, but that one should make sure (1) to have one’s liver checked regularly since EGCG had the potential to cause serious liver damage before one becomes aware of it and (2) to take EGCG in its purest available form since many popular green tea supplements contained lots of other ingredients which had been not studied.
As a result, I switched to the brand of EGCG they used in the PROMESA study, take only half the manufacturer’s recommended dose (240 mg/d instead of 480), bug my primary care physician to check my liver more often than usual, and hope for the best. (And drink some black tea on top of that.)
Best wishes,
zz
LikeLike
I think that the sentence you quoted from the press release … “The ability of bacterial curli to escape the intestine and reach the systemic circulation raises questions about a potential role in neurodegenerative processes.” … is intended to be read in the context of the sentence that immediately precedes it … “Recent research in Parkinson’s disease mouse models suggests that curli amyloid fuels neurodegeneration.”
Consequently, I didn’t get the impression that there is any “ongoing research behind the statement”. This latest research suggests that curli can escape the intestines, and previous research suggests that curli amyloid fuels neurodegeneration (perhaps via cross-seeding of alpha synuclein [1], or perhaps via triggering an immune response involving alpha synuclein [2]).
[1] Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans, Chen et al., Sci Rep. Oct 6 2016.
[2] A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity, Stolzenberg et al., Journal of Innate Immunity, June 27 2017.
LikeLike
You note that “there are several additional species that make amyloid proteins, including Streptococcus mutans, Staphlococcus aureus, Salmonella enterica, and Mycobacterium tuberculosis.”
You might want to add candida albicans to the above list. When candida overgrows in the gut into mycelial forms, the surface of that colony becomes stressed in ways that encourages the formation of amyloid proteins, as described in this article:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570177/
This suggests the possibility that candida may contribute to the aggregation of proteins in the gut, including alpha-synuclein, as described in this passage:
“Our gut presents the greatest opportunity for exposure to foreign organisms (our microbiota). It has been known since 2002 that the bacteria and fungi make functional extracellular amyloid proteins8. Bacterial amyloid proteins are highly conserved, are involved in biofilm formation and help the bacteria with invasion, host adhesion, and resistance to destruction8,9. The best studied bacterial amyloid protein is curli made by Escherichia coli and its key element, CsgA, has been found to contain amyloidogenic peptide repeat motifs shared by yeast [e.g., Candida–L.T.] and human prions10 and AS11. Cross–seeding, in which one amyloidogenic protein (curli, Tau, AB or AS, yeast prions, silk protein) causes another to adopt a beta-sheet structure has been documented12. There is excellent precedent for amyloid misfolding to be initiated through gut exposure (i.e., bovine spongiform encephalopathy, serum amyloid A amyloidosis13).
https://www.nature.com/articles/srep34477
Interesting that the article mentions amyloidosis, which would be the spread of amyloid proteins through the blood to various sites throughout the body. Because candida can *itself* poke holes in the gut and then spread through the blood to various sites around the body, and wherever it establishes itself it can also generate amyloids on its own surface.
So this seems like it could be an additional means for amyloids to spread throughout the body, in this case not as themselves but as candida, which then becomes its own little source of amyloid wherever it lands.
This raises in my beginner’s mind the question of whether the transmission of amyloid through the blood might be yet another way for aggregated alpha-synuclein to get into the brain, skipping the vagus nerve and the olfactory bulb and just crossing over the blood-brain barrier…? Or else, jumping onto some other part of the peripheral nervous system and traveling along axons into the brain just as it travels up the vagus nerve, but from a different starting point.
This article leads me to suspect that aggregated alpha-synuclein at least *might* cross from the blood across the blood-brain barrier, through astrocytes that implement the tight junctions of that barrier, and from there into neurons to which those astrocytes are transmitting oxygen from the blood. Here is an excerpt:
“For a-Syn transport from blood to ISF via the BBB, it is currently unknown whether brain endothelial cells possess the receptor-mediated or clathrin-mediated endocytosis, which could take up a-Syn molecules from the systemic circulation and serve as the source of a-Syn in brain parenchyma. We also do not know how a-Syn is transferred from cerebral endothelia to astrocytes prior to reaching neurons…”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4120720/
It also seems possible that candida itself might cross the blood-brain barrier and, once inside the brain, generate and expose amyloid proteins that could encourage the conversion of alpha-synuclein monomers already present there into aggregated forms. Last year some researchers at Baylor college probed that this happens–at least in mice.
LikeLike
Here is an excerpt from the Baylor 2019 study referenced above that shows not only that candida can cross the blood-brain barrier, but that once there is can generate and present amyloid proteins internal to the brain. Although, it is important to note, “in mice.”
“‘They injected yeast cells into the blood stream of mice and were surprised to discover that the yeast can cross the blood-brain barrier, a robust protective mechanism the brain employs to exclude all kinds of large and small molecules, as well as a number of microorganisms that can potentially damage the brain.’
“’We thought that yeast would not enter the brain, but it does,’ Professor Corry said.
“’In the brain, the yeast triggered the activity of microglia, a resident type of immune cell. The cells became very active ‘eating and digesting’ the yeast.’
“’They also produced a number of molecules that mediated an inflammatory response leading to the capture of the yeasts inside a granule-type structure inside the brain. We called it fungus-induced glial granuloma (FIGG).’
“The team also tested the animals’ memory in both yeast-infected and non-infected mice.
“The study authors found that infected mice had impaired spatial memory, which reversed when the infection cleared.
“The mice cleared the yeast infection in about 10 days; however, the microglia remained active and the FIGGs persisted well past this point, out to at least day 21.
“Intriguingly, as the FIGGs formed, amyloid precursor proteins accumulated within the periphery and amyloid beta molecules built up around yeast cells captured at the center of FIGGs. These amyloid molecules are typically found in plaques that are the trademark of Alzheimer’s disease.
“‘These findings suggest that the role fungi play in human illness potentially goes well beyond allergic airway disease or sepsis,’ Professor Corry said.
“‘The results prompted us to consider the possibility that in some cases, fungi also could be involved in the development of chronic neurodegenerative disorders, such as Alzheimer’s, Parkinson’s and multiple sclerosis. We are currently exploring this possibility.'”
http://www.sci-news.com/medicine/candida-albicans-brain-06787.html
LikeLike