
|
|

Source: Youtube
In 2002, deep brain stimulation (or DBS) was granted approval for the treatment of Parkinson’s disease by the US Food and Drug Administration (FDA). The historical starting point for this technology, however, dates quite far back…
Further back than many of you may be thinking actually…
In his text “Compositiones medicamentorum” (46 AD), Scribonius Largo, head physician of the Roman emperor Claudius, first suggested using pulses of electricity to treat afflictions of the mind.

Roman emperor Claudius. Source: Travelwithme
He proposed that the application of the electric ray (Torpedo nobiliana) on to the cranium could be a beneficial remedy for headaches (and no, I’m not kidding here – this was high tech at the time!).

Torpedo nobiliana. Source: Wikipedia
These Atlantic fish are known to be very capable of producing an electric discharge (approximately 200 volts). The shock is quite severe and painful – the fish get their name from the Latin “torpere,” meaning to be stiffened or paralysed, referring specifically to the response of those who try to pick these fish up – but the shock is not fatal.
Now, whether Largo was ever actually allowed to apply this treatment to the august ruler is unknown, and beyond the point. What matters here is that physicians have been considering and using this approach for a long time. And more recently, the application of it has become more refined.
What is deep brain stimulation?
The modern version of deep brain stimulation is a surgical procedure in which electrodes are implanted into the brain. It is used to treat a variety of debilitating symptoms, particularly those associated with Parkinson’s disease, such as tremor, rigidity, and walking problems.
First introduced in 1987, deep brain stimulation consists of three components: the pulse generator, an extension wire, and the leads (which the electrodes are attached to). All of these components are implanted inside the body. The system is turned on, programmed and turned off remotely.

Source: Ucdmc
The electrodes that are implanted deep in the brain are tiny, and the very tip of them has small metal plates (each less than a mm in width) which provide the pulses that will help mediate the activity in the brain.

DBS electrode tip. Source: Oxford
The electrode extends up into the leads (or extension wire) which continue up and out of the brain, across the top of the skull, down the neck and to the pulse generator which is generally located on the chest.

Xray image demonstrating the leads. Source: Fineartamerica
There are many different brands/types of pulse generators, but they all largely do the same basic function. They are titanium packets containing the electronics and power supply for the leads and electrodes. They are implanted in a subcutaneous spot, usually located under the collar bone on the chest.

Pulse generators. Source: Cambridge (also a good read on DBS)
The pulse generator is programmable (which the doctor usually does several weeks post surgery). A small hand held device is used to turn on/off the generator, generally by holding the device over it.

Remote control. Source: Cambridge
For more information on the topic, click here to read about a case report of deep brain stimulation implantation.
The following video (kindly provided online by Ken Miller) demonstrates the benefits of deep brain stimulation for a person with Parkinson’s disease:
Ken has also provided another video which demonstrates what happened to his tremor during the surgical procedure when they turned the stimulator on (watch what happens to his tremor from 50 seconds into the video!):
The patient is usually awake during most of the deep brain stimulation operation – a local anaesthetic keeps them blissfully unaware of what is happening on top of their heads – and they will be asked to perform motor movement tasks (such as tapping of the fingers) while the doctors adjust the electrodes.
How do the doctors know where to put the electrodes?
This is a good question.
How can the doctors possibly place a tiny metal wire into a minuscule region of a living brain (inside the skull) if they can not actually see the region? This is one of the key determinants of success with deep brain stimulation.
Before surgery, patients will be placed in a ‘stereotaxic frame’, which is a metal frame that is worn over the head for the surgery. This will provide the surgeons with reference points from which they can determine exactly where and how deep into the brain they need to go. They can calculate an exact location inside the head on a 3 dimensional basis by using the distances from various reference points (from a point on the front of the head, a point on the side of the head and a point on the top of the head) .

Stereotaxic frame – happy chap. Source: Mayfieldclinic
Once a person is inside the stereotaxic frame, surgeons will double down on the exactness of their calculations by actually having a look inside the head of that person. They do this using MRI brain imaging.
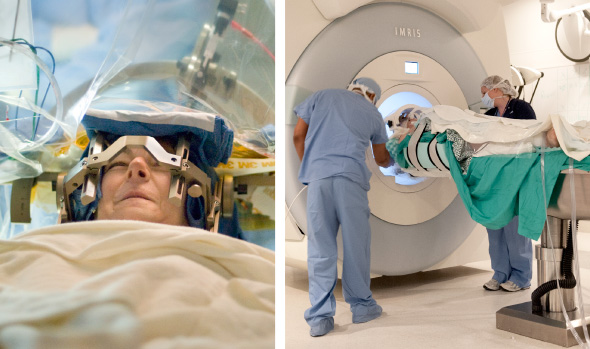
Stereotaxic frame and MRI imaging. Source: Consultqd
By imaging the brain, the doctors can determine the exact co-ordinates inside the brain that they want to target – in relation to the reference points on the stereotaxic frame. In this way, they can plan their surgery and (hopefully) increase the chances of success.

MRI image of the brain and where the electrodes will go.
Source: Parkinsonindia
How does deep brain stimulation actually work?
I have previously discussed how we initiate movement (Click here to read that post), and I will only briefly summarise the basics here.
Movement is largely controlled by the activity in a specific group of brain regions, collectively known as the ‘Basal ganglia‘.

The basal ganglia structures (blue) in the human brain.
Source: iKnowledge
The basal ganglia receives signals from the cortex regarding possible movements to make, and it processes that information before sending a signal on to another important participant in the regulation of movement: the thalamus.

A brain scan illustrating the location of the thalamus in the
human brain. Source: Wikipedia
The thalamus is a structure deep inside the brain that acts like the central control unit of the brain. Everything coming into the brain from the spinal cord, passes through the thalamus. And everything leaving the brain, passes through the thalamus. It is aware of most everything that is going on and it plays an important role in the regulation of movement. Importantly it is biased towards inhibiting movement – a signal from the basal ganglia has to be pretty strong, for the thalamus to give the green light and a particular movement can then be made.
Under normal circumstances, dopamine producing neurons release dopamine in the basal ganglia where it helps to mediate the local environment. It acts as a kind of lubricant for movement, the oil in the machine if you like. It helps to reduce the inhibitory bias of the basal ganglia.
Thus, with the loss of dopamine neurons in Parkinson’s disease, there is an increased amount of inhibitory activity. And as a result, the thalamus is kept in an overly inhibited state. And this is the reason why people with Parkinson’s disease have trouble initiating movement.

The workflow of the movement decision making process. Source: BJP
Now, as you can see from the image above, the globus pallidus is one of the main conduits of information into the thalamus. Given this pivotal position in the regulation of movement, the globus pallidus has been a region of major research focus for a long time.
It is also one of the sites targeted in deep brain stimulation therapy for Parkinson’s disease. Another target of deep brain stimulation therapy is the subthalamic nucleus which help to regulate the activity of the globus pallidus. By placing electrodes in one of these two brain regions (see image below), doctors can regulate the signal being passed from the basal ganglia to the thalamus and thus provide people with Parkinson’s disease relief from their motor-associated symptoms.

Two of the target sites for deep brain stimulation. Source: JAMA
But what do the electrodes do?
The electrodes will be programmed to release tiny electrical pulses that will help regulate the firing of the surrounding cells. By increasing the firing of those cells, deep brain stimulation reduces the inhibitory state of the thalamus.
The efficacy of deep brain stimulation is based on high frequency stimulation. Rates (or frequencies) below 50 pulses per second typically do not have an impact on stimulating the surrounding cells. In fact, low frequency can actually cause a stimulation-induced worsening of tremors. The most commonly used rates are between 130 and 185 pulses per second for Parkinson’s disease. And of course this is programmable and can be adjusted over time.
Are there alternatives to deep brain stimulation?
Yes there are.
Before deep brain stimulation was available, surgeons would sometime perform one of two procedures:
- Pallidotomy, involves a tiny electrical probe being placed into the globus pallidus, which is then heated to 80 °C (176 °F) for 60 seconds, destroying a small number of brain cells in that area.
- Thalamotomy, similar to the Pallidotomy but a small region of the thalamus is destroyed.
More recently, surgeons have been performing these sorts of procedures with non-invasive, high intensity focused ultrasound. Also known as magnetic resonance guided focused ultrasound, this procedure uses very intense ultrasound generated from multiple points but focused on one specific area.
The waves from those different points of emission are all in phase – that is to say their waves match. And all that ultrasound concentrated in one location generates a lot of heat at that focal point. That heat allows for the destruction of diseased or damaged tissue at that particular point of focus.

A schematic demonstrating the focused ultrasound technique.
Source: Ghanouni et al (2015)
I have previously gone into more detail on high intensity focused ultrasound (Click here to read that post).
There are several important issues, however, that need to be considered with regards to these procedures (high intensity focused ultrasound, Pallidotomy and Thalamotomy). They include are:
- The procedures are irreversible – once the damage is done, it cannot be repaired.
- Once performed, the effect is also unadjustable – there is no ‘volume control’ on the resulting effect.
And this is where deep brain stimulation has the advantage – it can be adjusted over time or turned off if it is having negative side effects.
Negative side effects?
There can be negative side effects to deep brain stimulation.
Firstly, there is the possibility that the electrodes can become displaced or dislodged from the specific location during or after the surgery. This misplacement is relatively easy to identify using brain imaging techniques. At best this can result in the individual having no benefit from the stimulation. More seriously, however, misplaced electrodes have been known to cause complications such as personality changes.

An example of misplaced electrodes. Source: Researchgate
There can also be complications resulting from the surgery, such as bleeding within the brain. Swelling of the brain as a result of the surgery can cause some disorientation or sleepiness. Longer term there have been reports of psychiatric side-effects, such as apathy issues or hallucinations (Click here for a good review of these potential side effects).
So what is the new research regarding deep brain stimulation?
The new research is rather amazing, because the scientists involved have managed to stimulate cells deep within the brain without the need of electrodes! No surgery at all.
Here is the research report:

Title: Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields
Authors: Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ, Cassara AM, Neufeld E, Kuster N, Tsai LH, Pascual-Leone A, Boyden ES.
Journal: Cell. 2017 Jun 1;169(6):1029-1041.
PMID: 28575667 (this article is OPEN ACCESS if you would like to read it)
The investigators from the Massachusetts Institute of Technology (led by Prof Ed Boyden – who has appeared once before on this website in a previous post – Click here to read that post) wanted to test what they called temporal interference stimulation. And yes, I know what you are going to ask next:
What is temporal interference stimulation?
Temporal interference stimulation takes advantage of the fact that neurons do not respond to electric fields with frequencies of 1,000 cycles per second or more. Generally, really high frequency electric fields pass through the brain without affecting neuronal activity.
However, if two fields are applied to the brain at the same time, both at high frequencies that differ by only a small amount (eg. one is field is 2000 cycles per second and the other is 2010 cycles per second, so the difference is 10 cycles per second), these fields will interfere with each other and this effect produces an ‘enveloping’ electric field within a particular region of the brain. This envelop (red in the image below) will excite the cells within it at the frequency which is the difference between the two fields (in our example: 10 cycles per second).

Source: Cell
In the envelope wave pattern (red) in the image above, you will see the high frequency waves overlap, but at the same time they produce the bigger wave shape that oscillates at a slower frequency (the red line running along the top of the high frequency red waves). And it is this ‘enveloping’ wave that stimulates the cells in the targeted area.
Cool trick huh?
The researchers validated the temporal interference concept using models, before then verified the effect in neurons in the brain of a living mouse – demonstrating that neurons could in fact be stimulated by the electric field envelope. Critically, the investigators demonstrated that temporal interference stimulation could be targeted to regions deep within the brain of living mice (such as the hippocampus), without stimulating neurons of the overlying cerebral cortex.

The hippocampus (white dots) under the cortex. Source: Guardian
In the image above, the cells in the hippocampus on one side of the brain (the white doted area) have been activated and they are now bright green, compared to the overlying cortex and the hippocampus on the other side of the brain.
Again, cool trick huh?
The investigators are already working on plans for future studies, which will utilise larger numbers of electrodes and multiple sets of envelopes (or interfering fields). Their hope is to be able to pinpoint even smaller regions of the brain for therapeutic purposes, or multiple regions of the brain (for research purposes). They are very curious to see how small a focal volume may be achieved.
Has non-invasive stimulation ever been tested in humans?
Yes….sort of.
But it’s not the kind of stimulation that I have described above. Several research groups have proposed ‘Transcranial direct current stimulation’ as a treatment for Parkinson’s disease. This procedure basically involves running a low current between two electrodes placed on either side of one’s head.

Doc Brown doing some stimulating research. Source: Pilerats
The current passes through the upper region of your brain (the cortex) and it does appear to have some effect on neural activity (even after the stimulation has ended). The duration of this change seems to depend on the length of stimulation as well as the intensity of stimulation.

Transcranial direct current stimulation. Source: Wikipedia
Has this ever been tested in people with Parkinson’s disease?
Yes, it has. In fact, it has been tested on all sorts of neurological conditions.
This was the first clinical study of this approach in Parkinson’s disease:

Title: Transcranial direct current stimulation for the treatment of Parkinson’s disease
Authors: Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, Hallett M.
Journal: J Neurol Neurosurg Psychiatry. 2010 Oct;81(10):1105-11.
PMID: 20870863 (This article is OPEN ACCESS if you would like to read it)
In this double blind, randomised study, 25 people with Parkinson’s disease were recruited. They were divided into two groups with 13 receiving transcranial direct current stimulation and the remaining 12 receiving a sham (pretend) stimulation. The transcranial direct current stimulation group had modest improvements in their gait features for a short period of time and improved in their bradykinesia (slowness of movement) for longer periods. But overall changes in Unified Parkinson’s Disease Rating Scale, reaction time, physical and mental well-being, and self-assessed mobility did not differ when compared to the control group.
Subsequent clinical studies have been less convincing (Click here for an example). There has also been a review of the clinical studies conducted on transcranial direct current stimulation and the general conclusion was that “there is insufficient evidence to determine the effects of transcranial direct current stimulation in reducing off time and on time with dyskinesia and for improving health-related quality of life, disability and impairment in patients with Parkinson’s disease” (Source).
So please don’t go wiring yourself up to the mains in an effort to try transcranial direct current stimulation at home – the evidence thus far certainly does not support it as a viable treatment for Parkinson’s disease.
So what does it all mean?
Right, so summing up: for over 15 years deep brain stimulation has provided people with Parkinson’s disease a programmable and adjustable method of controlling their motor-related symptoms. Recently, researchers from Boston have devised a new non-invasive method by which cells deep in the brain can be stimulated. This approach bypasses the need for surgery and permanent placement of electrodes into the brain. The researchers behind the new method “anticipate that it might rapidly be deployable into human clinical trials, as well as studies of the human brain”.
As exciting as this technology is, I suspect that this new non-invasive approach to deep brain stimulation is years away from getting to the clinic. And the reasons for this are purely technical. There are major challenges underlying the technology.
Firstly, the technology needs to be refined so that only tiny regions are targeted. At present, rather large areas are being affected by the stimulation. If this approach is going to be used for stimulating the globus pallidus or subthalamic nucleus, the focal point of this stimulation will need to be just millimetres across (unless an alternative, larger site of stimulation is proposed).
Second, any device applying this stimulation to a person’s head would need to be small and practical enough to allow individuals to move freely. And the device would need to be extremely secure on the head so that the focal point of stimulation in the brain is not moving around with every bump or fall.
If these obstacles can be overcome, then a kind of holy grail of ‘brain stimulation’ therapy will have been achieved. I will be keeping an eye out for what comes next here, but I will not be holding my breath. The refining of this technology may take some time (and of course, I’m very happy to be proved wrong on that!).
The banner for todays post was sourced from 2ndFriday
Simon, great job, very interesting topic and very exclamation of DBS with or without the need for surgery, congratulations! Frank
LikeLike
Thanks Frank. Prof Ed Boyden’s lab seem to be pretty confident that they can make this non-invasive approach a viable option. It will be interesting to see what they come out with. I am very curious. Hope all is well,
Simon
LikeLike