
|
For many people diagnosed with Parkinson’s disease, one of the scariest prospects of the condition that they face is the possibility of developing dyskinesias. Dyskinesias are involuntary movements that can develop after long term use of the primary treatment of Parkinson’s disease: Levodopa In todays post I discuss one experimental strategy for dealing with this debilitating aspect of Parkinson’s disease. |

Dyskinesia. Source: JAMA Neurology
There is a normal course of events with Parkinson’s disease (and yes, I am grossly generalising here).
First comes the shock of the diagnosis.
This is generally followed by the roller coaster of various emotions (including disbelief, sadness, anger, denial).
Then comes the period during which one will try to familiarise oneself with the condition (reading books, searching online, joining Facebook groups), and this usually leads to awareness of some of the realities of the condition.
One of those realities (especially for people with early onset Parkinson’s disease) are dyskinesias.
What are dyskinesias?
Dyskinesias (from Greek: dys – abnormal; and kinēsis – motion, movement) are simply a category of movement disorders that are characterised by involuntary muscle movements. And they are certainly not specific to Parkinson’s disease.
As I have suggested in the summary at the top, they are associated in Parkinson’s disease with long-term use of Levodopa (also known as Sinemet or Madopar).
Sinemet is Levodopa. Source: Drugs
How do dyskinesias develop in Parkinson’s disease?
There is a lot of debate over this topic, but there are some basic details that researchers generally tend to agree on.
Before being diagnosed and beginning a course of Levodopa, the locomotion parts of the brain in a person with Parkinson’s disease gradually becomes more and more inhibited. This increasing level of inhibition results in the slowness and difficulty in initiating movement that characterises this condition. A person with Parkinson’s may want to move, but they can’t.
In effect, they are akinetic (from Greek: a-, not, without; and kinēsis – motion).

Drawing of an akinetic individual with Parkinson’s disease, by Sir William Richard Gowers
Source: Wikipedia
Levodopa tablets provide the brain with the precursor to the chemical dopamine. Dopamine producing cells are lost in Parkinson’s disease, so replacing the missing dopamine is one way to treat the motor features of the condition. Simply giving people pills of dopamine is a non-starter: dopamine is unstable, breaks down too quickly, and (strangely) has a very hard time getting into the brain (it is blocked by the protective layer called the blood-brain-barrier). Levodopa, on the other hand, is very robust and has no problem entering the brain.
Once inside the brain, Levodopa is quickly converted into dopamine. It is changed into dopamine by an enzyme called DOPA decarboxylase, and this change rapidly increases the levels of dopamine in the brain, allowing the locomotion parts of the brain to function more normally.

The chemical conversion of L-dopa to dopamine. Source: Nootrobox
In understanding this process, it is important to appreciate that when an Levodopa tablet is consumed and Levodopa enters the brain, there is a rapid increase in the levels of dopamine. This ‘spike’ in the supply of dopamine will last for the next few hours, before the dopamine is eventually used up.
As the effects of the Levodopa tablet wear off, another tablet will be required. This use of multiple Levodopa pills across the day gives rise to a wave-like shape to the dopamine levels in the brain over the course of the day (see the figure below). The first pill in the morning will quickly lift the levels of dopamine enough that the individual will no longer feel akinetic. This will allow them to be able to function with normal controlled movement for several hours before the Levodopa begins to wear off. As the Levodopa wears off, the dopamine levels in the brain drop back towards levels that will leave the person feeling akinetic and at this point another Levodopa tablet is required.
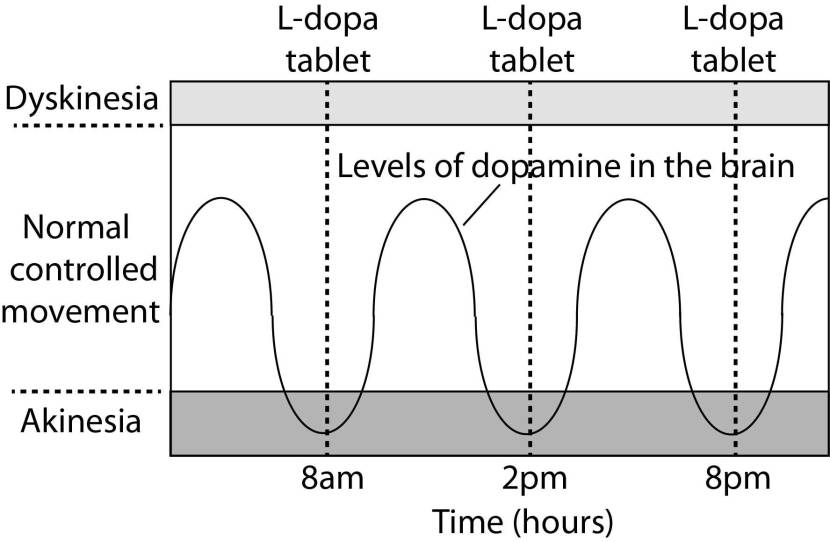
After several years of Levodopa use, many people with Parkinson’s disease will experience a weaker response to each tablet. They will also find that they have more time during which they will be unable to move (exhibiting akinesia). This is simply the result of the progression of Parkinson’s disease – Levodopa treats the motor features of the disease but only hides/masks the fact that the disease is still progressing.
To combat this shorter response time, the dose of Levodopa is increased. This will result in increasing levels of dopamine in the brain (as illustrated by the higher wave form over time in the image below). It will take more Levodopa medication induced dopamine to lift the individual out of the akinetic state.

This increasing of Levodopa dosage, however, is often associated with the gradual development of abnormal involuntary movements that appear when the levels of Levodopa induced dopamine are the highest.
These are the dyskinesias.
Are there different types of dyskinesias?
Yes there are.
Dyskinesias have been broken down into many different subtypes, but the two main types of dyskinesia are:
Chorea – these are involuntary, irregular, purposeless, and unsustained movements. To an observer, Chorea will look like a very disorganised/uncoordinated attempt at dancing (hence the name, from the Greek word ‘χορεία’ which means ‘dance’). While the overall activity of the body can appear continuous, the individual movements are brief, infrequent and isolated. Chorea can cause problems with maintaining a sustained muscle contraction, which may result in affected people dropping things or even falling over.
Dystonia – these are sustained muscle contractions. They often occur at rest and can be either focal or generalised. Focal dystonias are involuntary contractions in a single body part, for example the upper facial area. Generalised dystonia, as the name suggests, are contraction affecting multiple body regions at the same time, typically the trunk, one or both legs, and another body part. The intensity of muscular movements in sufferers can fluctuate, and symptoms usually worsen during periods of fatigue or stress.
I have previously discussed the current treatment options for dyskinesias (click here to see that post).
So what is the “alternative possible treatment” for dyskinesias you mentioned above?
This week the results of a phase III clinical trial were published in the journal Lancet:

Title: Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial
Authors: Anderson KE, Stamler D, Davis MD, Factor SA, Hauser RA, Isojärvi J, Jarskog LF, Jimenez-Shahed J, Kumar R, McEvoy JP, Ochudlo S, Ondo WG, Fernandez HH.
Journal: Lancet Psychiatry. 2017 Jun 28. pii: S2215-0366(17)30236-5.
PMID: 28668671
In this trial, the researchers recruited 298 people (aged 18-80 years) with tardive dyskinesia at 75 research centres in the USA and Europe.
What is tardive dyskinesia?
Tardive dyskinesias are involuntary, repetitive, jerky body movements – very similar to Levodopa-induced dyskinesias except that tardive dyskinesias are associated with the long-term use of neuroleptic medications (generally antipsychotics).

Neuroleptics. Source: PsychiatricResidents
First described in the 1950s shortly after the introduction of antipsychotic drugs, tardive dyskinesias are believed to result primarily from dopamine supersensitivity in the brain, particularly in the D2 dopamine receptor (Click here for a good review on this topic).
In their double-blind, randomised, placebo-controlled, phase III clinical trial, the researchers were testing a drug called Deutetrabenazine for its ability to reduced the involuntary movements in tardive dyskinesia.
What is Deutetrabenazine?
Also known as ‘Austedo’, Deutetrabenazine is a drug that is used in the treatment of involuntary movements associated with Huntington’s disease. It is a derivative of another drug called ‘Tetrabenazine’.
What is Tetrabenazine?
Tetrabenazine was the first approved treatment for Huntington’s disease. It helps in reducing the involuntary movements (called “Chorea“) that are characteristic of the condition. The precise mechanism of action of Tetrabenazine is unknown, but it is involved in a reversible depletion of dopamine, serotonin, norepinephrine, and histamine from nerve cells. We believe that Tetrabenazine is achieving this partly by binding to and inhibiting vesicular monamine transporters (VMATs).
VMATs are critically involved with collecting chemicals like dopamine and serotonin and packaging them into small sacks (called vesicles) within neurons. These vesicles are then transported to the tip of a neuron – where it meets another neuron, a region called the synapse – and the vesicle then releases its contents into that synaptic space. Some of the chemicals will make contact with receptors on the surface of the next neuron, and by binding to those receptors, they will help to pass on a signal. The chemicals that don’t achieve this task, however, will be taken back into the releasing neuron and be recycled or broken down and disposed of.

VMAT, vesicles and the synapse. Source: Nature
By inhibiting VMATs, Tetrabenazine basically stops dopamine from being released. If VMATs can’t put dopamine and serotonin into vesicles, the vesicles can’t release them into the synaptic space. And thus there is less dopamine actively being used in the brain. This is a very useful property when dealing with a condition like Huntington’s disease.
What is Huntington’s disease?
Huntington’s disease is an inherited neurodegenerative condition that affects 15 in every 100,000 people. It is caused by an expansion in the Huntingtin gene, which results in an abnormal “huntingtin” protein being produced and this gradually leads to cell death, and shrinkage of the brain. The exact mechanisms of this cell loss are unknown.
For those interested, this video provides a useful introduction to Huntington’s disease:
Huntington’s disease – like Parkinson’s disease – is a neurodegenerative disease. In some ways, Huntington’s is almost the mirror opposite of Parkinson’s because where PD has too little dopamine, HD basically has too much.
The cardinal feature of the Huntington’s disease brain is an almost complete loss of the structures making up the striatum (the caudate and the putamen). In the image below, a cross section of a normal brain is presented on the left and an HD brain on the right. In these images, you will note the reduced size of the striatum (indicated by the black arrow) in the HD brain when compared to the healthy brain.

Normal brain (left) and HD brain (right). Source: Prezi
The striatum plays an absolutely critical role in movement.
It provides an inhibitory force against the excited signals coming down from the motor cortex of the brain. Think of the motor cortex as a bunch of hyperactive, completely out of control toddlers, and the striatum as the sole parental figure. All of the toddlers are making demands/proposals and sending mixed messages about which movements the body should be making, and it is the job of the inhibiting striatum to gain control and decide which is the best course of action.
Under normal circumstances, dopamine neurons release dopamine in the striatum which helps to mediate the inhibitory environment. It acts as a kind of lubricant for movement, the oil in the machine if you like. It helps to reduce the inhibitory bias of the striatum, allowing for signals to actually pass through and movement to be initiated. With the loss of dopamine neurons in Parkinson’s disease, there is an increased amount of inhibitory activity in the striatum, which leaves an individual having a hard time to initiate normal movements.
In Huntington’s disease, however, there is a massive loss of cells in the striatum (see the image above), which results in a near complete loss of the inhibiting forces and too much dopamine floating around, and as a result there is less control of the chaotic set of signals coming from the “toddlers” in the motor cortex and being sent to the body. This results in the involuntary, jerky chorea movements associated with Huntington’s disease.
Here is a short video of a lady with Huntington’s chorea:
Ok, so these researchers took this drug that treats Huntington’s disease and they tested it on people with Tardive dyskinesias?
Exactly.
They randomly assigned their 298 patients into four groups, to receive a dose of placebo (n=74), deutetrabenazine 12 mg/day (n=75), 24 mg/day (n=74), or 36 mg/day (n=75). They found that both 24 mg/day and 36 mg/day of Deutetrabenazine treatment provided a significant reduction in levels of the dyskinesias associated with the condition. They concluded that dosing regimens of Deutetrabenazine could be used on an individualised and tailored basis for patients with tardive dyskinesia to help control their dyskinesias.
And soon after reading that research report this week, I started thinking:
If this drug helps in reducing the chaotic, jerky, involuntary movements of tardive dyskinesia (a condition resulting for long-term use of antipsychotic drugs), why wouldn’t it work for Levodopa-induced dyskinesias?
But hang on a second. That’s crazy! You are suggesting we use a drug that reduces dopamine levels in the brain… on people with Parkinson’s disease?!? How would reducing the level of dopamine being released also reduce the dyskinesias in a person with Levodopa-induced dyskinesias?
Yeah, I know!
And I fully understand how crazy and counter intuitive this idea sound. It really doesn’t really make sense on the surface.
And the only reason I am mentioning it, is because….. someone has already tried it!
And it seemed to have worked:

Title: Tetrabenazine improves levodopa-induced peak-dose dyskinesias in patients with Parkinson’sdisease.
Authors: Brusa L, Orlacchio A, Stefani A, Galati S, Pierantozzi M, Iani C, Mercuri NB.
Journal: Funct Neurol. 2013 Apr-May;28(2):101-5. doi: 10.11138/FNeur/2013.28.2.101.
PMID: 24125559 (This article is OPEN ACCESS if you would like to read it)
The researchers behind this study started with a basic idea: if levodopa-induced dyskinesias is partly due to a spike of excessive levels of dopamine in the brain, could Tetrabenazine help to reduce the dyskinesias by lowering dopamine levels? They tested this question in an open-label (meaning everyone knew the treatment they were receiving) clinical study involving 10 people with advanced Parkinson’s disease who had developed Levodopa-induced dyskinesias. All of these individuals were dyskinetic more that 20% of waking hours.
Now, simply reducing levels of Levodopa treatment doesn’t really work in alleviating dyskinesias, as lowering Levodopa treatment simply lowers levels of dopamine in the brain. This reduces all movements, not just the involuntary dyskinetic kinds. So, the investigators decided to try reducing dopamine after stimulating production (with Levodopa). They achieved this by treating the participants in the study with Tetrabenazine.
The study started with the participants having their Levodopa-induced dyskinesias evaluated in the morning by administration of a dose of Levodopa corresponding to 125% of their usual morning dose. Following this first evaluation, Tetrabenazine was administered twice a day and slowly increased up to 50 mg per day.
Each participant was evaluated again six weeks after the beginning of the Tetrabenazine treatment, and the researchers found a clear treatment effect on Levodopa-induced dyskinesias emerge at the 6 week evaluation (up to 45% reduction in the levels of dsykinesias; p<0.05). Importantly, the Parkinson’s disease motor features (as measured by the Unified Parkinson’s Disease Rating Scale (UPDRS)) for the whole group were not significantly modified. That is to say, the participants normal movement ability didn’t get worse with the treatment.
The participants in the study were also asked to keep dairies about their motor behaviour over the entire period of the study and analysis of this information revealed that the time spent experiencing disabling dyskinesias was reduced from 20% (at the start of the study or basal levels) to only 9% of waking hours. Remarkably, there was no change to their ON time – the period of normal motor functioning (see graphs below).

Reduction in disabling dyskinesias on Tetrabenazine (TBZ). Source: NCBI
The researchers were evidently pleased with this result, but cautioned that the current study only lasted 6 weeks and longer studies are required to assess its long-term benefits or risks of using Tetrabenazine to reduce Levodopa-induced dyskinesias.
Wow! This is great! Where can I get me some Tetrabenazine?
So, a couple words of sincere caution here.
First, this was an open label trial. A better test of the drug’s ability to reduce Levodopa-induced dyskinesias would have been in a double blind study (meaning both the participants and investigators don’t know who is receiving the drug or a placebo). Until such a trial has demonstrated beneficial effects, this approach should be considered strictly experimental.
And this designation is important, because the side effects of Tetrabenazine can be extremely serious. They include sedation, fatigue, insomnia, depression, suicidal thoughts, akathisia, anxiety and nausea. In addition, since Tetrabenazine is depleting the dopamine in the brain, this drug could actually worsen ones Parkinson’s features if not used correctly. Plus, this drug has never been well characterised in the elderly populations.
The makers of the drug recommend that Tetrabenazine should not be taken by anyone suffering suffering depression. Given that the drug also lowers serotonin levels in the brain, depression can result from using this drug. Nor should Tetrabenazine be taken in combination with, or within a minimum of 14 days of discontinuing with monoamine oxidase inhibitors (such as Rasagiline or Selegiline).
And finally, perhaps strangely, this idea of using Tetrabenazine to reduce Levodopa-induced dyskinesias hasn’t been followed up (to my knowledge, and I would be very pleased to be corrected on this).
What does it all mean?
So summing up: Recently a clinical trial found the a dopamine depleting drug which is used to treat Huntington’s disease, had beneficial effects in treating the involuntary, jerky movements associated with the condition tardive dyskinesia. In this post, I have presented some preliminary clinical evidence suggesting that that same drug, Tetrabenazine, could potentially be used in treating Levodopa-induced dyskinesias in Parkinson’s disease.
This post was intended more for the Parkinson’s research community than the general readership, to bring attention to this alternative approach to treating dykinesias. Given that there are currently so few treatment options for this debilitating aspect of Parkinson’s disease, one feels that all possible approaches should be thoroughly investigated and tested. Despite the potential side effects of Tetrabenazine use, this drug could possibly help people suffering Levodopa-induced dyskinesias. Thus, I am presenting this information here in the hope that the idea will be further investigated.
UPDATE: 26-07-2017
Just to note that at the time of writing I was unaware of another VMAT inhibitor called Ingrezza (also known as Valbenazine) has been approved this year by the FDA (Click here for the press release) for use in treating tardive dyskinesias having reached its primary end points in a phase III trial (Click here to see the trial research report). Perhaps another drug that could possibly help people suffering Levodopa-induced dyskinesias.
FURTHER UPDATE: 30-09-2017
Today the U.S. Food and Drug Administration (FDA) approved AUSTEDO® (deutetrabenazine) tablets for the treatment of tardive dyskinesia in adults (Click here to read more about this).
Still no news on applying this drug to Levodopa-induced dyskinesias.
EDITORIAL NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. Please speak with your medical physician before attempting any change in an existing treatment regime.
Simon, thank you for providing all the information I have been reading and enjoying it. Also sometimes I post on health unlocked site for everybody else with Parkinson’s to read. I also have Parkinson’s. Mary
LikeLike
Hi Mary,
Thanks for your comment and for sharing the info. Please feel free to share any information posted on this website. All of the material on this website is licensed under a Creative Commons Attribution 4.0 International License, so you can do whatever you like with it!
Kind regards,
Simon
LikeLike
Just as a quick FYI for anyone who may see this article…On August 24, 2017, the FDA approved a drug called Gocovri (amantadine) that is specifically for patients that experience levodopa-induced dyskinesia. The drug, made by Adamas Pharm. will be available late 2017 or early 2018.
LikeLike
Hi Bob,
Thanks for your comment. It is true that the FDA recently approved Gocovri for L-dopa induced dyskinesias, but the cost of this new drug (which is simply a slow release version of the generic drug, amantadine) is expected to be between between $10,000 and $30,000 a year. This is slightly higher than generic amantadine, which can be currently bought for as little as 64 cents per pill. At two pills a day, a yearly supply costs around $500. And amantadine has long been used off-label by doctors for treating dyskinesia. But thanks for pointing out Gocovri. It may be something worth exploring for those affected by dyskinesias.
Kind regards,
Simon
LikeLike
Hi Simon,
Thanks for your reply. Yes, I over looked that off-label scripts for amantadine are somewhat common. However, I also know that generic amantadine has a short window of effectiveness, requiring multiple doses during the day. Here’s where Gocovri may be useful as it is taken before bedtime and is effective in treating dyskinesia the entire following day. It remains to be seen whether this more convenient and effective formulation will be worth the extra cost. Thanks for your work. Bob
LikeLike