|
This week, biotech firm Prothena published the results of their Phase I safety and tolerance clinical trial of their immunotherapy treatment called PRX002 (also known as RG7935). Immunotherapy is a method of artificially boosting the body’s immune system to better fight a particular disease. PRX002 is a treatment that targets a toxic form of a protein called alpha synuclein – which is believed by many to be one of the main villains in Parkinson’s. In today’s post, we will discuss what immunotherapy is, review the results of the clinical trial, and consider what immunotherapy could mean for the Parkinson’s community. |
Source: uib
I have previously mentioned on this website that any ‘cure for Parkinson’s’ is going to require three components:
- A disease halting mechanism
- A neuroprotective agent
- Some form of cell replacement therapy
This week we got some interesting clinical news regarding the one of these components: A disease halting mechanism.
The Phase I results of a clinical trial being conducted by a company called Prothena suggest that a new immunotherapy approach in people with Parkinson’s is both safe and well tolerated over long periods of time.
The good folks at Prothena Therapeutics. Source: Prothena
What is immunotherapy?
Immunotherapy is a method of boosting the body’s immune system to better fight a particular disease.
It involves utilising the immune system of your body, and artificially altering it to target a particular protein/disease-causing agent that is not usually recognised as a pathogen (a disease causing agent).
Immune cells attacking a cancer cell. Source: Lindau-nobel
It is potentially a very powerful method of treating a wide range of medical conditions, and the research on immunotherapy is particularly robust in the field of oncology (‘cancer’). Numerous methods of immunotherapy have been developed for cancer and are currently being tested in the clinic (Click here to read about the many clinical trials now under way).
Many approaches to immunotherapy against cancer. Source: Bloomberg
One of the most promising of these cancer-based immunotherapy approaches is called CART immunotherapy (or chimeric antigen receptor (CAR) T-cell immunotherapy).
This video explains how CART immunotherapy works:
Interesting, but how is immunotherapy being used against Parkinson’s?
One of the big theories about how Parkinson’s progresses involves the idea that a toxic form of the Parkinson’s-associated protein, alpha synuclein, could be being passed from cell to cell.
And as this toxic version of alpha synuclein is absorbed by each new healthy cell, it starts causing trouble in that healthy cell and this results in clustering (or aggregation) of protein, which is believed to lead to the appearance of Lewy bodies in those previously healthy cells.
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells – see the image below where alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.
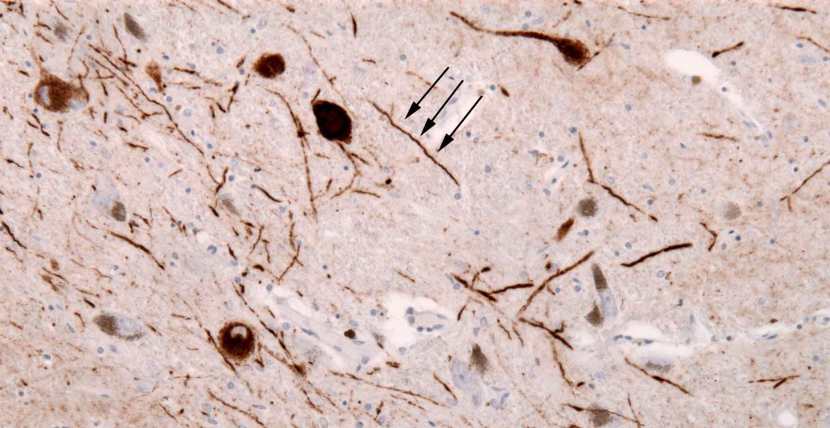
Examples of Lewy neurites (stained in brown; indicated by arrows). Source: Wikimedia
When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure. Alone, it will look like this:
Alpha synuclein. Source: Wikipedia
By itself, alpha synuclein is considered a monomer, or a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of Monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein give rise to the aggregations of protein that we refer to as Lewy bodies:
Parkinson’s associated alpha synuclein. Source: Nature
And it also believed that the oligomer and fibril forms of alpha synuclein protein are being passed from cell to cell, and ‘seeding’ protein aggregation in new cells. And this is how the condition may be slowly progressing.
Is there any evidence of this transfer of the alpha synuclein protein?
So back in the 1990s, there were a series of clinical trials of cell transplantation conducted on people with Parkinson’s. The idea was to replace the cells that have been lost to the condition (Click here to read a previous post about cell transplantation). Many of the individuals who were transplanted have now passed away by natural causes and their brains have been examined post-mortem.
One very interesting finding from the analysis of those brains is that some of the cells in the transplants have Lewy bodies in them (up to 10% of transplanted cells in one case – Click here to read the research report on that case).
Above are photos of neurons from the post-mortem brains of people with Parkinson’s that received transplants. White arrows in the images above indicate lewy bodies inside transplanted cells. Source: The Lancet
This finding suggested to researchers that somehow this neurodegenerative condition is being passed on from the Parkinson’s affected brain to the healthy transplanted cells.
And researchers have proposed that the toxic form of the Parkinson’s-associated protein alpha synuclein may be the guilty party in this process (Click here to read more on this idea and the evidence for it).
So how is immunotherapy being applied to Parkinson’s?
One way of dealing with this problem of cell-to-cell transfer of the toxic form of alpha synuclein is to grab it as it is being passed between the cells, and remove it from the body.
This idea has given rise to a series of ongoing clinical trials that are using antibodies which target the toxic form of the alpha synuclein protein.
What are antibodies?
Antibodies are Y-shaped proteins that the immune system naturally and continuously produces to identify anything in the body that is ‘not self’ (that is, not a normally occurring part of you – think of viruses, bacteria, etc).

Monoclonal antibodies. Source: Astrazeneca
Antibodies act like alert flags for the immune system. When antibodies bind to something, they alert the immune system to investigate and potentially remove. Each antibody targets a very specific structure, while ignoring everything else.
In this fashion, antibodies are a very powerful method of removing items from the body that are causing trouble or not wanted.
And researchers have adapted this natural system for Parkinson’s using immunotherapy approaches. Currently, immunotherapy is being tested in Parkinson’s in two ways:
- Active immunisation – this approach involves the body’s immune system being encouraged to target the toxic form of alpha synuclein. The best example of this is a vaccine – a tiny fragment of the troublesome pathogen is injected into the body before the body is attacked, which helps to build up the immune systems resistance to the pathogen (thus preventing the disease from occurring).
- Passive immunisation – this approach involves researchers designing antibodies themselves that specifically target a pathogen (such as the toxic form of alpha synuclein, while leaving the normal version of the protein alone). These artificially generated antibodies can then be injected into the body.
Immunotherapy. Source: Acimmune
Several biotech companies are currently clinically testing immunotherapy approaches for Parkinson’s. An Austrian company called AFFiRiS is trialing an active immunisation method with their “Parkinson’s vaccine” called ‘AFFITOPE® PD01A’ (Click here to read a previous post on this topic).

The big pharmaceutical company Biogen is also testing an immunotherapy treatment for Parkinson’s which involved the passive immunisation technique. Their drug, called BIIB054 is currently in Phase II clinical trials for Parkinson’s (Click here to read a previous post on this topic and click here to learn more about the Phase II ‘SPARK study’ trial).

Which of these approaches is Prothena using?
Prothena therapeutics has taken the second approach (Passive immunisation) with their treatment PRX002.
They have designed an antibody that specifically targets the aggregated form of alpha synuclein, and this treatment is intravenously injected into individuals with Parkinson’s (who have chosen to take part in the clinical trials).
And what are the new results they published this week?
They are the results of the Phase I safety and tolerance study of PRX002:
Title: Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-α-Synuclein Monoclonal Antibody, in Patients With Parkinson Disease: A Randomized Clinical Trial.
Authors: Jankovic J, Goodman I, Safirstein B, Marmon TK, Schenk DB, Koller M, Zago W, Ness DK, Griffith SG, Grundman M, Soto J, Ostrowitzki S, Boess FG, Martin-Facklam M, Quinn JF, Isaacson SH, Omidvar O, Ellenbogen A, Kinney GG.
Journal: JAMA Neurol. 2018 Jun 18. doi: 10.1001/jamaneurol.
PMID: 29913017 (This report is OPEN ACCESS if you would like to read it)
In this Phase Ib study, the researchers recruited 80 mild to moderate idiopathic Parkinson’s (Hoehn and Yahr stage 1-3; average age was 58 years) from July 2014 to September 2016. These participants were randomly assigned to receive either placebo or PRX002 (25 people received placebo and 55 were administered PRX002). Most of the participants used dopaminergic medications before starting the study (96.0% placebo vs 80.0% PRX002), did not have any family history of Parkinson’s (84.0% placebo vs 78.2% PRX002), and did not have a personal history of rapid eye movement sleep behavioural disorder (88.0% placebo vs 72.7% PRX002).
The study found that multiple intravenous injections of PRX002 every 4 weeks for 52 weeks was safe and well tolerated. There were no serious or severe treatment associated events reported. The ‘elimination half-life’ (the period when blood concentrations of the drug have halved) of the treatment was approximately 10.2 days. Infusions of PRX002 resulted in significant dose- and time-dependent reductions of free alpha synuclein in the blood (in some cases up to 97%).
The average levels of PRX002 getting across the blood-brain barrier – the protective membrane that surrounds our central nervous system – was measured at 9 weeks. The levels of PRX002 in cerebrospinal fluid was found to increase with dose, and was found to be approximately 0.3% of the levels found in the blood. While this is only a small fraction of the total amount of PRX002 in the body, the researchers at Prothena Therapeutics are confident (based on computer modelling from the preclinical models and Phase 1 studies) that levels of PRX002 in the brain meet what is needed for target engagement of the aggregated forms of alpha synuclein.
Unfortunately, there is no validated assessment available today for determining levels of aggregated alpha synuclein so it was not possible for Prothena to determine if there was any reduction of this form of alpha synuclein in the blood or brain fluid. They did note, however, that there was no statistically significant change in spinal fluid levels of free alpha synuclein was observed in the PRX002 treated groups, suggesting that in the brain at least the normal, un-aggregated form of alpha synuclein was not being tartgeted by PRX002.
The report concluded that results suggest that PRX002 is well tolerated and safe in people with Parkinson’s. And all of the data collected has provided useful information for designing and conducting the ongoing Phase II trial to assess whether PRX002 (also referred to as RG7935) demonstrates any benefit in people with Parkinson’s during a period of 52 weeks.
Ongoing Phase II trial?
Yes, while being very cautious with this new class of experimental treatment, Prothena Therapeutics is wasting little time in pressing forward with the clinical testing of PRX002.
The Phase II trial of the drug (now referred to as RO7046015) is called the PASADENA study (listed on the Clinicaltrials.gov as NCT03100149).
This new study involves two parts. Part 1 is a randomised, double-blind, placebo-controlled, three-arm study which will enrol approximately 300 people with Parkinson’s (all less than 2 years since diagnosis) to evaluate the efficacy and safety of PRX002 in people with Parkinson’s over 52 weeks. Participants will be randomly assigned to one of three groups (1500 mg or 4500 mg of PRX002, or placebo treatment). The treatments will be administered via intravenous infusion once every 4 weeks. One complicating aspect of this first part of the study is that eligible participants must not be on any dopaminergic therapy, and they must not be expected to require dopaminergic therapy for the 52 weeks of Part 1 of the study – identifying subjects that fit this criteria may slow recruitment.
Part 2 of this Phase II clinical trial is a 52-week blinded extension phase in which participants from the placebo group of the study will be re-randomly assigned into one of two active doses on a 1:1 basis. This means that all participants will be on active treatment. Participants who were originally assigned to an active dose will continue at that same dose level for the additional 52 weeks. In part 2 of the study, participants will be allowed to use dopaminergic therapies (if required).
We are not expecting the results of this trial until 2020/21, although if the treatment works very well the FDA can halt the trial and fast-track approval.
So what does it all mean?
Boosting the immune system to target proteins associated with neurodegenerative conditions, represents a powerful new approach towards tackling diseases like Parkinson’s. This week a US-based biotech firm published the results of their Phase I clinical study which demonstrated that their treatment – PRX002 – was safe and well tolerated in 55 people with Parkinson’s.
The company is now conducting a Phase II efficacy clinical study to determine whether PRX002 can actually have a beneficial effect on slowing or halting Parkinson’s. Here at the SoPD we are watching this trial very carefully.
Fingers and toes are crossed.
The banner for today’s post was sourced from Prothena











Fingers crossed indeed. Prothena claims Prx002 has 400x affinity for misformed a-syn. So hopefully that 0.3 % in the CSF is getting the job done. Wish they would publish progression data….
Need a time machine.
LikeLike
Indeed Double. That time machine would be very useful – could also go back in time with some lottery numbers, win big and fund more PD clinical trials/research with the winnings.
That is an interesting note regarding Prothena’s claim. From conversatations with them, the company is certainly confident that enough of the drug is getting into the brain.
Kind regards,
Simon
LikeLike
@simon
since reading the article published on 29 December last year, I have been womdering whether I have MSA. . This has now been confirmed by my consultant. It is good to know that there are treatments being developed.
Are there any UK based trials? How long will it be before a treatment becomes availabele?
Keep up the good work Smon, publishing the Science in a very understandable form.
KW
LikeLike
Hi KW,
Sorry to hear about the misdiagnosis. The nature of MSA is different between individuals (some cases have lived for 20 years post diagnosis). And there are certainly clinical trials focused on MSA (both research and experimental treatment). On the research side of things there is the PROSPECT-M study here in the UK (https://clinicaltrials.gov/ct2/show/NCT02778607?cond=MSA&draw=3&rank=20). It is a longitudinal study seeking to identify biomarkers for Progressive Supranuclear Palsy (PSP), Cortico-Basal Degeneration (CBD) and Multiple System Atrophy (MSA) – the less common variants of Parkinsonisms. While also exploring other treatment-based trials you may be interested in talking with the researchers involved in the PROSPECT-M study.
With regards to experimental treatment clinical trials, the MSA coalition have a webpage for finding trials (https://www.multiplesystematrophy.org/resources/find-msa-clinical-trials) and the Michael J Fox foundation also have a great search engine (https://foxtrialfinder.michaeljfox.org/pages/find-trials/default.aspx/?keywords=multiple%20system%20atrophy&distance=25&webbased=2&archive=False).
I hope this helps.
Kind regards,
Simon
LikeLike
Simon
Thank you.
KW
LikeLike
So, if this is halting the disease, we wouldn’t expect any improvement or worsening of symptoms.
As you say, Simon, fingers crossed!
And to the Peasant Farmer, my prayers are with you
LikeLike
Hi Garvil,
With these disease-halting approaches, they will hopefully leave folks where they are (that is to say, the individual won’t progress any further). This would be great for a person who have just been diagnosed or may be about to be diagnosed, but not so great for someone who has had the condition for a considerable period of time. And this is why the other components of a ‘cure’ for Parkinson’s are required (firstly, A neuroprotective agent to protect the remaining cells and provide a supportive/nurturing environment for the third component: some form of cell replacement therapy to replace cells that have been lost). And as I said near the top of this post, there are clinical trials ongoing for both of these additional components as we speak.
Fingers crossed for them too!
Simon
LikeLike
@Gavril thanks
kW
LikeLike
Hi Simon,
Very interesting write-up–just noticed it. Out of curiosity, is there a reason you haven’t gone into similar detail regarding results from Biogen’s immunotherapy drug (BIIB054)? I believe it’s also in Phase 2.
Full disclosure: I was one of the patients in the BIIB054 Phase 1 trial and wonder how prospects for it are perceived by researchers not involved with the trials, esp. relative to Prothena’s drug.
LikeLike
Hi David,
Thanks for your comment and for reminding me about BIIB054. I wrote a post back in April about the Phase I trial results (https://scienceofparkinsons.com/2018/04/25/biib054/), but I note this morning that I never inserted that post into the site map (or search engine), which means that folks would not have found it if they were looking for information about BIIB054. The situation has now been remedied – much appreciated. I’ll also provide links to that post in this post.
Biogen’s alpha synuclein targeting antibody, BIIB054, is now in Phase II. The SPARK study (https://www.thesparkstudy.com/) kicked off in December 2017, with the first participant being dosed in January of this year (http://www.neurimmune.com/newsartikel/25012018-in-january-2018-biogen-dosed-the-first-patient-in-the-phase-2-spark-study-of-biib054-anti-alpha-synuclein-antibody-in-parkinsons-disease.html). It is very difficult to compare these immunotherapy treatments as we have so little information at present regarding the antibodies. We will have to wait until the Phase II results are announced before any judgements can really be made.
Kind regards,
Simon
LikeLike
Thanks, Simon.
LikeLike