|
Here at the SoPD, we are primarily interested in disease modification for Parkinson’s. While there is a great deal of interesting research exploring the causes of the condition, novel symptomatic therapies, and other aspects of Parkinson’s, my focus is generally on the science seeking to slow, stop or reverse the condition. At the start of each year, it is a useful practise to layout what is planned and what we will be looking for over the next 12 months. Obviously, where 2020 will actually end is unpredictable, but an outline of what is scheduled over the next year will hopefully provide us with a useful resource for better managing expectations. In this post, I will try to lay out some of what 2020 holds for us with regards to clinical research focused on disease modification for Parkinson’s. |

Lord Robert Baden-Powell. Source: Utahscouts
My old scout master once looked around our horse shoe, making eye contact with each of us, before asking the question:
“When did Noah build the ark?”
My fellow scouts and I looked at each other – confused. Did he want an exact date?!?
The scout master waited a moment for one of us to offer up some idiotic attempt at an answer – thankfully no one did – before he solemnly said:
“Before the rain”
It was one of those childhood moments that made little sense at the time, but comes back to haunt you as an adult when you are looking at what the future may hold and trying to plan for it.
# # # # # # # # # # #
Today’s post is our annual horizon scanning effort, where we lay out what is on the cards for the next 12 months with regards to clinical research focused on disease modification in Parkinson’s.
 Source: Rand
Source: Rand
We will also briefly mention other bits and pieces of preclinical work that we are keeping an eye on for any news of development.
To be clear, this post is NOT intended to be an exercise in the reading of tea leaves – no predictions will be made here. Nor is this a definitive or exhaustive guide of what the next year holds for disease modification research (if you see anything important that I have missed – please contact me). And it should certainly not be assumed that any of the treatments mentioned below are going to be silver bullets or magical elixirs that are going to “cure” the condition.
In the introduction to last year’s outlook, I wrote of the dangers of having expectations (Click here to read that post). I am not going to repeat that intro here, but that the same message applies as we look ahead to what 2020 holds.
 Source: Unitystone
Source: Unitystone
In fact, it probably applies even more for 2020, than it did for 2019.
2020 is going to be a busy year for Parkinson’s research, and I am genuinely concerned that posts like this are only going to raise expectations. My hope is that a better understanding of where things currently are and what is scheduled for the next 12 months will help in better managing those expectations. Please understand that there is still a long way to go for all of these experimental therapies.
All of that said, let’s begin:
As stated in the intro, I am going to focus primarily on clinical trials of potentially disease modifying experimental therapies in this post, as a broader discussion of ‘all Parkinson’s research in 2020’ is too greater task.
And in keeping with previous years outlooks, I am going to frame this discussion around the idea that:
Any ‘curative therapy’ for Parkinson’s is going to require three core components:
- A disease halting mechanism
- A neuroprotective agent
- Some form of restorative therapy
Now, the bad news is (as far as I am aware) there is no single treatment currently available (or being tested) that can do all three of these things. By this I mean that there is no disease halting mechanism therapy that can also replace lost brain cells. Nor is there a restorative therapy that stop the progression of the condition.
That statement can obviously be read as bad news, but it shouldn’t.
Let me explain:
A curative therapy for Parkinson’s is going to need to be personalised to each individual, with varying levels of each of the three component listed above. It will be a multi-modal approach designed for each individual’s needs.
 Making things personal. Source: Flickr
Making things personal. Source: Flickr
By this I mean, there is a great deal of heterogeneity (or variability) between individuals with regards to their symptoms and the amount of time that they have had the condition. Some folks are more tremor dominant, while others do not experience tremor at all. Likewise, some individuals have only just been diagnosed, while others have lived with the condition for many years.
The treatment needs of each individual will be different, and thus what we will require is different amounts of the disease halting mechanism component, the neuroprotection component, and the restorative therapy components for each affected person.
Now the good news is that there is considerable clinical research currently being conducted on each of these three components. And we will now explore what is happening in each of these components and discuss what is scheduled for 2020 (I have underlined “finished in 2020” where a trial is completing this year – there are quite a few!).
|
SPEACIAL NOTE: Before we start, this website is the personal blog of the deputy director of research at The Cure Parkinson’s Trust. The Trust is a UK registered charity which is a supporter of many of the clinical trials mentioned in this post. For the purposes of full disclosure, where appropriate I will note the Trust’s involvement. In addition, I would like to thank Parkinson’s advocates Sue Buff, Gary Rafaloff and Kevin McFarthing for the efforts they put into maintaining their respective databases of Parkinson’s clinical trials (Sue & Gary maintain the PDTrialTracker website, while Kevin keeps the “hope list“). This post would not be possible without those amazing resources. Also, a special thanks also to Jeff K for pointing out all my typos in the first version of this post! |
Let’s now consider the first component:
1. A disease halting mechanism
Parkinson’s is a progressive neurodegenerative condition. Thus, the first and most critical component of any ‘cure’ for Parkinson’s involves a treatment that will slow down or halt the progression of the condition.
This can be done either directly or indirectly.
The direct approach
The direct approach involves treatments that specifically targets the underlying biology of the condition.
A direct approach in halting Parkinson’s, however, requires a fundamental understanding of how the condition is actually progressing. And if we are honest, we are not there yet – we still do not have a solid grasp of how Parkinson’s progresses over time. In addition, this may vary between individuals. It is gradually being agreed that rather than being a single ‘disease’, Parkinson’s may actually be a ‘syndrome’ – that is, a collection of conditions that share similar symptoms.
We do, however, have some very good educated guesses as to what is happening, and there are numerous clinical trials focused on attempts at “direct approaches” to halting Parkinson’s. For example, there is a protein called alpha synuclein which is associated with Parkinson’s.
It is believed to be passed from cell to cell, seeding the condition in each cell as it goes, and thus, it may underly the progression of Parkinson’s. Researchers have targeted this protein as a means of slowing/halting Parkinson’s.
The direct approach: Alpha synuclein
One of the direct approaches being employed against alpha synuclein is a method called immunotherapy.
Immunotherapy involves boosting the body’s immune system to target specific toxic agents in the body. In the case of Parkinson’s, this approach is primarily being focused on different forms of alpha synuclein.

Antibodies. Source: Astrazeneca
The immunotherapy approach uses antibodies, which are Y-shaped proteins that act like alert flags for the immune system. Once enough antibodies bind to a particular object, the immune system will dispose of it. Antibodies target very specific structures, while ignoring everything else.
In Parkinson’s, the immunotherapy approaches are primarily involving antibodies that target the alpha synuclein protein. By tagging the alpha synuclein as it is being passed from one cell to another, and allowing the immune system to remove it, researchers hope to slow down the progression of Parkinson’s.
Immunotherapy can be conducted in two ways:
- The body’s immune system can be encouraged to develop its own antibodies that target the toxic form of alpha synuclein (using active immunisation in the form of a vaccine); or
- Researchers can design antibodies themselves that specifically target the toxic form of alpha synuclein (while leaving the normal version of the protein alone), and then inject those antibodies into the body (passive immunisation)
 Immunotherapy. Source: Acimmune
Immunotherapy. Source: Acimmune
There are now numerous biotech firms testing passive immunotherapy approaches in the clinic for Parkinson’s, but in reality there are two main studies in the immunotherapy for Parkinson’s that everyone has their eyes on (simply because of their advanced progress in the clinical trial process).
The first is the PASADENA study. This is a Phase II clinical trial of an alpha synuclein targeting immunotherapy (called Prasinezumab – formerly called RO7046015 & PRX002) being conducted by the pharmaceutical company Roche and biotech firm Prothena Biosciences.
This study involves two parts.
Part 1 is a randomised, double-blind, placebo-controlled, three-arm study which will enrol approximately 300 people with Parkinson’s (all less than 2 years since diagnosis) to evaluate the efficacy and safety of RO7046015 in people with Parkinson’s over 52 weeks. Participants will be randomly assigned to one of three groups (1500 mg or 4500 mg of Prasinezumab, or placebo treatment). The treatments will be administered via intravenous infusion once every 4 weeks.
 Part 2 of this Phase II clinical trial is a 52-week blinded extension phase in which participants from the placebo group of the study will be re-randomly assigned into one of two active doses on a 1:1 basis (Click here to read more about this study).
Part 2 of this Phase II clinical trial is a 52-week blinded extension phase in which participants from the placebo group of the study will be re-randomly assigned into one of two active doses on a 1:1 basis (Click here to read more about this study).
Part 1 of the Pasadena study was scheduled to complete in December 2019, with Part 2 finishing in February 2021.
The second main immunotherapy study is the SPARK study being conducted by the Pharmaceutical company Biogen.
 This is also a 2-year Phase II clinical trial that will test Biogen’s alpha synuclein targeting immunotherapy treatment BIIB054 in an estimated group of 311 people with Parkinson’s. In the first year of the study, participants in the study will randomly assigned to monthly infusions of 3 different doses of BIIB054 (250mg, 1250mg, or 3500mg) or a placebo treated group (Click here to read more about this study and click here to read a SoPD post about the Phase I Biogen study results). At the start of year two, members of the placebo group will switch to receive BIIB054 treatment as well.
This is also a 2-year Phase II clinical trial that will test Biogen’s alpha synuclein targeting immunotherapy treatment BIIB054 in an estimated group of 311 people with Parkinson’s. In the first year of the study, participants in the study will randomly assigned to monthly infusions of 3 different doses of BIIB054 (250mg, 1250mg, or 3500mg) or a placebo treated group (Click here to read more about this study and click here to read a SoPD post about the Phase I Biogen study results). At the start of year two, members of the placebo group will switch to receive BIIB054 treatment as well.
 The one-year placebo-controlled treatment period is scheduled to end in May 2020, and the overall trial is planned to complete in summer 2021.
The one-year placebo-controlled treatment period is scheduled to end in May 2020, and the overall trial is planned to complete in summer 2021.
Both of these studies are not expected to report any results in 2020 (beyond an announcements of Part 1 completions for both studies). But they are leading the pack of immunotherapy programmes for Parkinson’s which have clinical trials that are scheduled to finish in 2020, including:
- Astrazeneca‘s immunotherapy treatment called MEDI1341 (currently in Phase I safety testing in healthy volunteers; scheduled to finish in November 2020 (Click here to read more about that study).
- Lundbeck‘s immunotherapy treatment called Lu AF82422 (which is being developed in collaboration with Genmab), which is currently in Phase I safety testing in both healthy volunteers and people with Parkinson’s; scheduled to finish in June 2020 (Click here to read more about this).
In addition to these clinical programs, the pharmaceutical company AbbVie has an immunotherapy treatment called BAN0805/ABBV-0805 (which is being developed in collaboration with BioArctic Neuroscience). Phase I safety testing was initiated in 2019, and is scheduled to finish in mid 2021 (Click here to read more about this). These two companies have two additional alpha synuclein-targeting immunotherapy treatments called PD1601 & PD1602 which are also in development.
Now you may recall that we mentioned two types of immunotherapy above – passive and active. The clinical trials we have discussed above is passive immunotherapy.
In addition to these passive immunotherapy treatments, there are also two companies that are testing active immunotherapy treatment in Parkinson’s. These are vaccines for Parkinson’s, which targets the toxic form of alpha synuclein.
The company with the most advanced vaccine program is called AFFiRiS.

And they are clinically trialing a vaccine treatment called ‘AFFITOPE® PD01A’ (Click here to read a previous SoPD post on this topic).
At the time of writing this post, we have not had any updates from AFFiRiS since May 2018 (Source). Thus, in 2020, we here at the SoPD HQ are really hoping to get some news/updates regarding ongoing follow-ups of participants in their Phase I clinical studies, AND to hear word of the initation of a larger Phase II clinical trial – particularly because their last press release had some ambigous sentences regarding study outcomes which still need to be clarified – see the bottom of this post to read about this).
A second company developing a vaccine against alpha synuclein is United Neuroscience‘s (UB-312).
This biotech company is focused on developing a novel class of vaccines that are fully synthetic (they call them ‘endobody vaccines‘) and can train the body to treat/prevent neurological condtions. They are currently conducting a Phase I safety/tolerability trial of UB-312 in healthy volunteers and in participants with Parkinson’s. The results of this study are scheduled for June 2021 (Click here to read more about this trial).
|
SMALL SIDE NOTE HERE: Some readers have questioned the untility of immunotherapy in neurodegenerative conditions like Parkinson’s, given the inability of this approach to demonstrate any effect in Alzheimer’s. All of the immunotherapy clinical trials in Alzheimer’s thus far have failed to demonstrate any impact on the course of that condition. But in late 2019, the Pharma company Biogen reported that one of their huge Phase III clinical trials did have a positive outcome and they are now petitioning the US FDA for market authorisation for their treatment, aducanumab (Click here to read more about this). This news has revived interest in immunotherapy approaches for neurodegenerative conditions, and it will be interesting to see what news 2020 brings in this domain. Here at the SoPD we make no assumptions about what drugs might or might not work on large populations. Rather, if three people in every 100 respond to a particular experimental therapy, we are interested in what is unique about those individuals and what can that tell us about their Parkinson’s. |
One of the acknowledged limitation of the immunotherapy approaches, is the low amount of antibody actually accessing the brain. In the Alzheimer’s immunotherapy trials, only 1-3% of the treatment in the blood gets into the brain. This is due to a protective membrane surrounding our brains, called the blood brain barrier, which limits entry of most drugs/proteins.
These limited amounts of immunotherapy treatment still allowed for the clearance of the Alzheimer’s targeted protein (beta amyloid) so it can be assumed that it should also be enough to be able to reduce levels of alpha synuclein in the Parkinson’s immunotherapy clinical trials.
But these immunotherapy trials will have limited ability to actually affect alpha synuclein within cells (remember, they are tagging and grabbing the protein as it is being passed between cells). This situation has led a growing number of biotech companies to develop small molecules that can enter and target alpha synuclein inside of cells.
One example of this is a drug called NPT088, which is being developed by Proclara Biosciences.
 Formerly called NeuroPhage Pharmaceuticals, Proclara is clinically testing NPT 088 which can apparently break down any aggregated protein (beta amyloid, Tau, alpha synuclein, etc). It was being tested in individuals with probable Alzheimer’s (Click here to read more about this) – the company has stated on their website that the results would be applicable to Parkinson’s (Source). The trial finished in February 2019, and the results have recently been published (Click here to read more about this). In 2020, we look forward to hearing news of further development of this drug.
Formerly called NeuroPhage Pharmaceuticals, Proclara is clinically testing NPT 088 which can apparently break down any aggregated protein (beta amyloid, Tau, alpha synuclein, etc). It was being tested in individuals with probable Alzheimer’s (Click here to read more about this) – the company has stated on their website that the results would be applicable to Parkinson’s (Source). The trial finished in February 2019, and the results have recently been published (Click here to read more about this). In 2020, we look forward to hearing news of further development of this drug.
Another biotech firm developing a small molecule to target alpha synuclein is NeuaroPore Therapies.
They are clinically testing a small molecule inhibitor of alpha synuclein called NPT520-34. Phase I testing in healthy individuals finished in September 2019, so we have looking forward to hearing the results of this study in 2020 (Click here to read more about this trial).
NeuroPore Therapies have out-licensed another small molecule alpha synuclein inhibitor called NPT200-11 to the pharma company UCB.
This drug has also been Phase I tested (Click here to read more about that trial). The study was completed (Click here to read the press release), and we look forward to learning more about the future development of this drug in 2020.
Another experimental drug, called ENT-01, is currently being clinically tested a company called Enterin Inc.
Between 2017-2018, the company conducted the RASMET study, which was a Phase I safety clinical trial of ENT-01 (Click here for the details about this trial and click here to read a SoPD post on this topic). The issue with ENT-01 compared to other molecules targeting alpha synuclein is that it does not cross the blood brain barrier. Thus, Enterin are focusing their clinical trial on Parkinson’s-associated constipation – can this drug reduce alpha synuclein aggregation in the gut and alleviate complaints like constipation.
The results of the Phase I RASMET study have been published (Click here to read them and click here to read the press release), and the company is currently conducting a Phase IIa ‘KARMET’ clinical study of ENT-01, which is scheduled to finish in October 2020 (Click here to read more about this study).
In addition, while ENT-01 does not cross the blood brain barrier, the company has another compound Trodusquemine which does get into the brain. So it would be pleasing to hear news regarding the development of trodusquemine (and perhaps the initiation of a Phase I clinical trial) in 2020.
One of the more novel alpha synuclein-targted approaches for Parkinson’s is being clinically tested by the biotech company Yumanity.
 This company is developing Stearoyl CoA desaturase inhibitors – these are a class of drugs that have been reported in preclinical research to reduce alpha synuclein-associated toxicity (Click here to read a SoPD post on this topic). Yumanity have recently been Phase I clinically testing their first drug, YTX-7739, in 48 healthy individuals (Click here to read more about this trial). That trial was scheduled to finish in late 2019, so we may (hopefully) hear news of the results and whether they are moving to Phase II in 2020.
This company is developing Stearoyl CoA desaturase inhibitors – these are a class of drugs that have been reported in preclinical research to reduce alpha synuclein-associated toxicity (Click here to read a SoPD post on this topic). Yumanity have recently been Phase I clinically testing their first drug, YTX-7739, in 48 healthy individuals (Click here to read more about this trial). That trial was scheduled to finish in late 2019, so we may (hopefully) hear news of the results and whether they are moving to Phase II in 2020.
A topic of great interest to many readers of the SoPD has been the sweetner Mannitol, which – after some interesting preclinical results – was crowd sourced into a patient-led online study by a group called Clinicrowd.
The results of that online study have been published (Click here to read a previous SoPD post about this topic), and have stimulated a Phase I clinical trial in Israel (Click here to read more about that trial). That trial is scheduled to finish in December 2020).
And finally a new entrant to the small molecule inhibitors of alpha synuclein field is Anle138b which is being developed by the biotech firm MODAG.
 Phase I clinical testing of this drug was initiated in 2019, and is scheduled to complete in October 2020 (Click here to read more about this). Anle138b currently being targeted at Multiple System Atrophy – a neurodegenerative condition similar to Parkinson’s – but the company is keen to test the molecule in Parkinson’s as well (Click here to read a previous SoPD post about this topic).
Phase I clinical testing of this drug was initiated in 2019, and is scheduled to complete in October 2020 (Click here to read more about this). Anle138b currently being targeted at Multiple System Atrophy – a neurodegenerative condition similar to Parkinson’s – but the company is keen to test the molecule in Parkinson’s as well (Click here to read a previous SoPD post about this topic).
A lot of alpha synuclein trials finishing this year – see what I mean by 2020 being a busy year?
|
OTHER DEVELOPMENTS TO WATCH FOR IN 2020 REGARDING ALPHA SYNUCLEIN: In March 2018, a biotech company called Arvinas entered into a research agreement with The Silverstein Foundation, focused on efforts to develop a blood brain barrier-penetrant, alpha synuclein-targeting PROTAC. Proteolysis targeting chimera (or PROTAC) technology is a system of degrading intracellular proteins which Arvinas is now clinically testing (Phase I testing of two PROTACs, ARV-110 and ARV-471, suggests they are safe and well tolerated (source)). In 2020, we will be hoping to hear news about the development of the alpha synuclein-targeting PROTAC. Another PROTAC-like approach for neurodegenerative conditions is being attempted by a company called C4 Therapeutics. This biotech firm uses a degradation tag (dTAG) for targeting pathogenic proteins that need to be disposed of (Click here to read recent research about this). Just a couple of days into 2019, C4 Therapeutics and the pharmaceuticals company Biogen announced a strategic collaboration to investigate the use of C4T’s novel protein degradation technology to discover and develop new treatments for neurodegenerative conditions, like Parkinson’s and Alzheimer’s (Click here to read the press release). We will be hoping for news on this front as well is 2020. Another company I am watching is Skyhawk. This company signed a deal with Biogen in early 2019 to develop treatments for neurological disorders. They use small molecule therapeutics to selectively target and correct RNA expression. Will be looking out for interesting news from this collaboration in 2020. A biotech firm called Annovis (formerly QR Pharma) are currently clinically testing ANVS-401 (also known as Posiphen) in Alzheimer’s (Click here to read more about that clinical trial). Interestingly, Posiphen has been reported to suppress the translation of both Alzheimer’s-associated amyloid precursor protein and alpha synuclein (Click here to read more about this). The Alzheimer’s clinical study was scheduled to complete in December 2019. It will be interesting to see the results of that trial in 2020. Another small biotech taking an interesting approach to alpha synuclein is Nitrome Biosciences. Nitrome has identified a alpha-synuclein nitration and aggregation — a hallmark of Parkinson’s — is catalyzed by a newly identified enzyme that we have termed Synuclein Nitrase. Nitrome’s drugs are intended to inhibit Synuclein Nitrase One other alpha synuclein-related biotech the SoPD will be keeping an eye on is Wren Therapeutics. Founded in 2016, perhaps in 2020 we will learn about what they have been up to with regards to misfolded proteins. Prof Sir Chris Dobson (who sadly passed away in 2019) was one of their co-founders. |
The direct approach: LRRK2
Not all of the direct approaches to slowing Parkinson’s involve targeting alpha synuclein protein. In fact, there are some cases of Parkinson’s that do not involve any accumulation of alpha synuclein at all.
Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’) is a Parkinson’s-associated protein, which becomes hyperactive in some cases of the condition. This over-active form of the protein is believed to be associated with the neurodegeneration seen in some cases of Parkinson’s.
To counter the over-active form of this protein in the carefully balanced environment of a cell, researchers have been developing inhibitors of this protein. The hope is that by inhibiting LRRK2, we will be able to slow down/halt the cell death and stablise the course of Parkinson’s.
Leading the pack in the race to develop LRRK2 inhibitors is a biotech firm called Denali Therapeutics.
Set up by a group of ex-Genentech scientists, Denali is already clinically testing two LRRK2 inhibitors: DNL-151 and DNL-201 (Click here to read a previous SoPD post about Denali’s efforts). A Phase Ib study of DNL-151 was initiated in 2019 (click here to read more about this), and the study is scheduled to finish in 2020 (Click here to read more about this study). And a Phase Ib trial of DNL-201 was registered in late 2018 (Click here to read more about this study). It too is scheduled to finish in late 2020.
I will also be curious to learn more in 2020 about a collaboration that was announced between Denali Therapeutics and SIRION Biotech, which is a company focused on developing viral vector’s for gene therapy (Click here to read the press release). Very curious.
|
SMALL SIDE NOTE HERE: LRRK2 is known to promote RIPK1 activation (Source). Receptor-interacting protein kinase 1 (RIPK1) is a protein involved with multiple cellualr pathways leading to inflammation or cell death. And there is evidence for involvement of RIPK1 in Parkinson’s (Example; also see this review). Interestingly, in early 2019 Denali and the pharma company Sanofi initiated two Phase I clinical trials of their RIPK1 inhibitor, DNL747. One of these studies was in Alzheimer’s and the other was in motor neurone disease (or ALS), and both were scheduled to complete in late 2019 (Click here and here to read more about these studies). If determined to be safe, it could be interesting to further explore the idea of RIPK1 inhibition in Parkinson’s (particularly if upstream LRRK2 inhibition is difficult to tolerate long-term – no evidence of this to date though). |
Another company interested in LRRK2 is the pharmaceutical company Biogen. In 2019, the biotech firm initiated a new clinical trial programme focused on LRRK2 in Parkinson’s.
 Working in collaboration with the biotech firm Ionis Pharmaceuticals, Biogen is going to clinically test a different kind of LRRK2 inhibition.
Working in collaboration with the biotech firm Ionis Pharmaceuticals, Biogen is going to clinically test a different kind of LRRK2 inhibition.
The companies have developed BIIB094 – an antisense oligonucleotide targetting LRRK2. Antisense oligonucleotides are a method of inhibiting RNA rather than proteins – this means that this drug blocks LRRK2 RNA rather than the subsequent protein (Click here to read a previous SoPD post about this approach). A Phase I clinical trial of BIIB094 was registered in late 2019. Called the “REASON study”, it is not scheduled to complete until early 2022, but I am mentioning it here to bring readers attention to it if they are interested in being involved (Click here to read more about this).
|
OTHER DEVELOPMENTS TO WATCH FOR IN 2020 REGARDING LRRK2: One major pharmaceutical company that has taken an interest in LRRK2 is GlaxoSmithKline. The company signed a 4 years partnership with the DNA analysis company 23andMe in 2018. As part of that deal, GSK is contributing its LRRK2 inhibitor program, and they may be hoping to use 23andMe’s database of people who know their LRRK2 genetic status. Such access would help their Parkinson’s clinical program enroll participants quicker in any planned clinical trial of their LRRK2 inhibitor (Click here to read more about this). In addition, GSK initiated a LRRK2 observation clinical study at King’s College in London (Click here to read more about this). It will be interesting to see how things develop in 2020. And still on the LRRK2 front, I will be looking out for new entrants in the area of LRRK2 inhibitors, for example Cerevel Therapeutics. This is a biotech firm that was started by Bain Capital and the Pharmaceutical company Pfizer (Click here to read more about this). Cerevel has taken on many of the neuroscience treatments that Pfizer was clinically testing until it shut down their neuroscience division in early 2018. In addition to those clinically tested assets, Cerevel have also quietly added ‘LRRK2 inhibitor’ to their preclinical ‘lead development’ area of research (Click here to read more about this). A biotech company called E-Scape Bio is also developing a LRRK2 inhibitor program (Click here to read more about this). Perhaps we will learn more about this in 2020. And finally, it will be interesting to learn in 2020 what has developed from the Servier and Oncodesign collaboration to develop novel LRRK2 inhibitors (Click here to read more about this). |
This post is very quickly starting to feel like a never ending shopping list of clinical trials.
Still with me?
Is it interesting?!?
I hope so – we have a loooong way to go!
We continue:
Additional direct approaches
In addition to alpha synuclein and LRRK2, there are a number of other Parkinson’s associated proteins that are being targeted by biotech firms for clinical development. Most are still in preclinical development, but worthy of mention here in order to provide a broad view of the different approaches being taken to tackle Parkinson’s.
First, there is Mitokinin.
This biotech firm is developing a drug that aims to increase the activity of active-form the Parkinson’s-associated protein PINK1. They recently were awarded a grant from the Michael J Fox Foundation to take their drug forward (Click here to read the press release).
There is also Vincere bioscience (Vincere comes from the Latin for “To win“).
Started in October 2018, the company was spun out from NeuroInitiative, LLC a computational biology company focused on identifying drug targets for Parkinson’s. They also were awarded a grant from the Michael J. Fox Foundation to accelerate a PARKIN activator program (Click here to read more about this). PARKIN is a protein that has many functions, and disruption of it is associated with increased risk of developing Parkinson’s.
Another company developing a PARKIN-focused approach is a Korean company called Cellivery.
This biotech firm is developing a cell-permeable form of PARKIN (iCP-Parkin), which has been reported to promote preservation of dopamine neurons in models of Parkinson’s (Click here to read more about this).
One of the newer entrants to the world of Parkinson’s research is a biotech firm that popped up on the radar in 2019. Curax is developing a molecule that helps in the removal of a protein called Miro1.
In 2019, researchers reported that a failure to effectively remove Miro1 was apparent in a large proportion of cells collected from both people with Parkinson’s and those at high risk of developing the condition – raising the possibility of a potential biomarker. But the researchers then conducted a drug screen and identified a small molecule that promotes Miro1 degradation, and they found that this drug could rescue mulitple Parkinson’s models (Click here to read a previous SoPD post on the topic).
This is where activities currently lie with regards to the direct approach to slowing or halting Parkinson’s progression.
We will now shift our attention to the:
The indirect approach
While the direct approach to halting disease progression requires a solid understanding of the underlying biology of Parkinson’s, indirect methods do not.
The indirect approach does not necessarily target the underlying mechanism of the condition, but rather attempts to slow progression by improving the health of affected cells, and allowing them to function better in the face of whatever is driving Parkinson’s.
One way we can improve the health of cells (and potentially slow the progression of Parkinson’s) is to enhance their ability to clear (or dispose of) toxic proteins. This approach generally involves boosting the waste disposal systems of the cell – in this manner, the cells can break down and dispose of excess proteins (like alpha synuclein) inside the cell before they have a chance to builds up and becomes toxic.
Cellular waste disposal/recycling is referred to as autophagy.
Helping cells to clean themselves up by boosting waste disposal systems, we will hopefully make the cells healthier and function better. And by limiting the build up of proteins – like alpha synuclein – these experimental therapies may help to slow down the progression of Parkinson’s.
As you shall see below, there are numerous clinical trials currently testing different therapies targeting different aspects of the autophagy process.
Some cases of Parkinson’s (up to 20%) are associated with genetic variations in particular sections of DNA (or genes). Parkinson’s is not considered a genetic disease, but tiny errors in the genetic code can increase one’s chances of developing the condition.
Genes provide the instructions for making some of the proteins, and some of the genes affected in Parkinson’s are involved in autophagy-related processes. One of the most common of these genes is called GBA. This gene produces an enzyme (called Glucocerebrosidase) that breaks down specific proteins.
The indirect approach: GBA-based approaches
Given that genetic variants in the GBA gene are the most common in Parkinson’s, a great deal of research is being conducted on this particular gene, with multiple clinical trials testing GBA-based therapies.
And in 2020, we are looking forward to seeing the results of one of those trials, which involves a clinically available drug called Ambroxol:

Ambroxol. Source: Skinflint
In 2020, we will see the publication of the “Ambroxol in Disease Modification in Parkinson Disease” (or AIM-PD) clinical trial, which has been supported by the Cure Parkinson’s Trust, the Van Andel Research Institute (USA) and the John Black Charitable Foundation (Click here to read more about the details of this study).
Ambroxol is a commonly used treatment for respiratory diseases. It promotes the clearance of mucus and eases coughing. It also has anti-inflammatory properties, reducing redness in a sore throat. But there is evidence that this drug can increase the levels of the GBA protein (Glucocerebrosidase) in models of Parkinson’s (Click here to read a SoPD post on this).
We are looking forward to seeing the results of this study published early in 2020.
There is also a second Ambroxol study, which is being conducted by the Lawson Health Research Institute (and the Weston Foundation) in Canada. This is a phase II, 52 week trial of Ambroxol in 75 people with Parkinson’s Disease Dementia (Click here to read more about this trial). In this randomised, double blind study, two doses of Ambroxol were tested – a high dose (1050 mg) and a low dose (525 mg) – as well as a placebo treated group. This study is scheduled to finish in late 2021.
Another GBA-related approach involves a drug called Venglustat (formerly known as GZ/SAR402671 & Ibiglustat) which is being conducted by the biotech company Sanofi Genzyme.
This drug is being tested in a clinical trial called “MOVES-PD” (Click here to read more about the trial) is a phase II clinical study that will be involve in two parts:
- A dose escalation study to determine safety in early-stage GBA-associated Parkinson’s.
- A randomised, double blind study of efficacy of Venglustat, as compared to placebo in early-stage GBA-associated Parkinson’s
Preclinical results involving this treatment approach look promising (Click here to read some of the research on this), and while this study is not expected to report until after 2022, we did see results presented at the Alzheimer’s/Parkinson’s 2019 meeting and Movement Disorder Society meeting last year (2019), suggesting that Venglustat is safe and well tolerated.
Another clinical trial of a GBA-targeted therapy that we will be seeking news about in 2020 was conducted by a biotech firm called Lysosomal Therapeutics.
 This company has now completed Phase I clinical studies of their experimental drug LTI-291, which is an activator of the Glucocerebrosidase enzyme (Click here to read a previous SoPD post on this topic). The results have not been published yet, but the company’s website suggests that the “study established an excellent safety and tolerability profile in normal human volunteers” (Click here to read more).
This company has now completed Phase I clinical studies of their experimental drug LTI-291, which is an activator of the Glucocerebrosidase enzyme (Click here to read a previous SoPD post on this topic). The results have not been published yet, but the company’s website suggests that the “study established an excellent safety and tolerability profile in normal human volunteers” (Click here to read more).
In 2020, we will also be looking for news from a biotech firm called Prevail Therapeutics.
This company is initiating an ambitious gene therapy clinical trial for GBA-associated Parkinson’s. Gene therapy involves treating medical conditions with DNA rather than drugs. The “PROPEL” trial will attempt to introduce a normal version of the GBA gene into the brain, allowing the cells to correct any autophagy disfunction (Click here to read more about this trial and click here to read a SoPD post on the this trial).
Another company developing a GBA gene therapy approach is AVROBIO.
 The company is focused on Type 1 Gaucher disease at present, but have indicated that their gene therapy treatment (called AVR-RD-02) could be used in Parkinson’s (Source).
The company is focused on Type 1 Gaucher disease at present, but have indicated that their gene therapy treatment (called AVR-RD-02) could be used in Parkinson’s (Source).
We will also be watching out for the results of a recent Phase I clinical trial of a drug called ESB1609, which is being developed by E-scape Bio.
ESB1609 is a novel, orally administered, brain-penetrant, selective sphingosine 1-phosphate 5 (S1P5) receptor agonist. S1P5 provides a powerful target that is upstream to some of the autophagy-related (lysosomal) deficits associated with conditions like GBA-associated Parkinson’s (Click here to read more about this). The first clinical trial was a single dose study, and the company is seeking to start a larger multi-dose study in early 2020.
|
OTHER DEVELOPMENTS TO WATCH FOR IN 2020 REGARDING GBA: One interesting GBA-related development from 2019 was a research report in October, highlighting a new small-molecule modulator of glucocerebrosidase (the GBA protein). The drug was called S-181 (Click here to read more about this and click here to read the press release). A new company Surmount Bio has been set up to explore & develop S-181 further. It will be interesting to see how this effort progresses in 2020. Another company Oxyrane was awarded a grant from the Michael J. Fox Foundation in 2018 to develop an “Innovative Glucocerebrosidase enzyme therapy as a disease-modifying systemic treatment for Parkinson’s disease” (Click here to read more about this). Will be keeping an eye out for developments here. We will also be looking out for any news from Gain Therapeutics regarding their GBA-associated Parkinson’s research program. They are using their site-directed enzyme enhancement therapy platform to identify compounds that are able to stabilize misfolded proteins AND restore the protein’s enzymatic activity. The Michael J. Fox Foundation and The Silverstein Foundation funded them in early 2019 (Source). Probably a bit early to expect much new data, but we all love surprises. It will also be interesting to learn more about Chamishi Therapeutics. Launched in September 2019 (by The Silverstein Foundation and by Q-State Biosciences), Chamishi will focus on developing antisense oligonucleotide therapies (similar to the BIIB094 treatment for LRRK2 discussed above) for people with Parkinson’s who also carry GBA genetic mutations (Click here to read more about this). |
Another ‘indirect’ approach to slowing Parkinson’s involves a class of drugs called c-Abl inhibitors. These molecules started life as cancer drugs, but they are now being re-purposed for Parkinson’s.
c-Abl is a protein that becomes activated in cells that are stressed and inhibiting it can boost autophagy. Multiple independent labs have demonstrated that this is a worthy target for Parkinson’s (Click here to read a review on this topic).
The indirect approach: c-Abl inhibitors
The first c-Abl inhibitor to be clinically tested in Parkinson’s was Nilotinib and some of the results were made available in late 2019.
 Nilotinib. Source: William-Jon
Nilotinib. Source: William-Jon
Following evidence suggesting beneficial effects in models of Parkinson’s and a small open label Phase I pilot study (Click here to read more about this), two larger double-blind clinical trials were initiated: PD Nilotinib and NiloPD.
PD Nilotinib, was conducted at Georgetown University in Washington DC (Click here for the more details about this study), and in late 2019 the investigators reported that the drug was safe at lower doses, but “no significant differences were seen in motor and nonmotor outcomes between the nilotinib groups and the placebo group” (Click here to read more about this).
The NILO-PD study was a multi-centre study which also finished in 2019, and while the results are still to be published, the co-ordinators have announced that while the drug was tolerated at the doses tested, there was no evidence of clinical benefits (Click here to read more about this).
(The Cure Parkinson’s Trust was a supporter of the NILO-PD study)
|
SMALL SIDE NOTE HERE: I have had a number of readers requesting a SoPD post discussing the results of the two Nilotinib clinical trials. The results of the NILO-PD study are not yet published, however, so to avoid any confusion, I am going to wait until those results are also available before writing that post. This will allow us to look at the two studies (and sets of results) in parallel. I will, however, say that both studies have indicated that very little of the drug appears to have accessed the brain (less than 1% of blood levels, due to the blood brain barrier), which suggests that this drug may not be the best test of c-Abl inhibition in the brain. Other clinical trials using c-Abl inhibitors specifically designed for neurological conditions (discussed immediately below) will be better tests of this class of drug for Parkinson’s. |
Other c-Abl inhibitors are being developed for neurological conditions, chief among these for Parkinson’s is K0706, which is being developed by Sun Pharma Advanced Research Company (or SPARC).
 In 2019, SPARC initiated an international Phase II, randomised, double-blind, placebo-controlled clinical trial of K0706 in 500+ people with early Parkinson’s (Click here to read more about this study). This study is expected to finish in early 2021.
In 2019, SPARC initiated an international Phase II, randomised, double-blind, placebo-controlled clinical trial of K0706 in 500+ people with early Parkinson’s (Click here to read more about this study). This study is expected to finish in early 2021.
Another c-Abl inhibitor being targeted at Parkinson’s is FB-101, which is being developed by the biotech firms 1ST Biotherapeutics and Neuraly.
FB-101 has now entered Phase I clinical testing in healthy volunteers (Click here to read more about that study) and that study is scheduled to finish mid 2020.
In 2020, we will also hopefully hear news about another c-Abl inhibitor called Radotinib, which is being developed by South Korean firm Ilyang Pharmaceutical.
Radotinib is currently in phase III testing for chronic myeloid leukemia (Click here to learn more about that trial).
And another biotech company, Inhibikase Therapeutic, will hopefully provide news regarding the clinical testing of their Nilotinib-like drug, called IkT-148009 in 2020 (Click here to read more about this).
In February 2019, the company filed two Investigational New Drug (IND) applications with the US FDA to initiate human clinical trials of IkT-148009 (Click here to read the press release), but we haven’t heard anything since. There are whispers in the wind that Phase I testing is underway, but we are waiting for confirmation of this from the company.
An additional autophagy-based therapy being clinically tested is a drug called RTB101, which is being developed by resTORbio Inc.
RTB101 is a TORC1 inhibitor. TORC1 is a master regulator of autophagy, and by inhibiting it, the waste disposal system of a cell is boosted. The Phase I ResTorbio clinical study is being conducted in New Zealand, and the trial is scheduled to finish in 2020 (Click here to read more about this trial and click here to read a SoPD post on this trial).
|
OTHER DEVELOPMENTS TO WATCH FOR IN 2020: Another company that is developing TORC1 inhibitors is Navitor Pharmaceuticals. It is still early days for Navitor, but one to watch perhaps. Similarly, Casma Therapeutics is developing drugs that activate the calcium channel TRPML1 to promote autophagy. They were awarded a Michael J Fox Foundation grant in 2019 to test TRPML1-activating drugs in models of Parkinson’s. We will be interested to learn of new developments from both companies in 2020. One additional autophagy-related biotech company worth watching in 2020 is Samsara Therapeutics. Started in early 2019, following the publication of a research report (Click here to read that report), Samsara is working with an autophagy-stimulating Japanese herb called ashitaba that is consumed on the island of Okinawa. Does it have mystical powers? Only time will tell. |
Another indirect approach to slowing the progression of Parkinson’s has recently been proposed which focuses around an anti-inflammatory approach.
The indirect approach: Anti-inflammatory approaches
The first of these anti-inflammatory approaches involves inhibiting the inflammasome.
Inflammasomes are multi-protein formations, present inside of cells in your body, and they can amplify the immune response to damage or a pathogen. Recent preclinical research suggests that blocking the inflammasome can rescue models of Parkinson’s (Click here to read a recent SoPD post on this topic)
Researchers have also reported that the Parkinson’s-associated protein alpha synuclein can promote the activation of inflammasomes in the immune cells of the brain: the microglia.
This has given rise to the development of NLRP3 inhibitors (NLRP3 is a key protein involved in inflammasomes). The first of these inhibitors to reach the clinic is Inzomelid, which is being developed by a biotech firm called Inflazome.

In mid 2019, the company initiated Phase I clinical testing in healthy volunteers (Click here to read more about this). That trial is scheduled to finish in early 2020, and inflazome are interested in clinically testing their drug in neurodegenerative conditions like Parkinson’s.
|
OTHER DEVELOPMENTS TO WATCH FOR IN 2020 REGARDING THE INFLAMMASOME: Another biotech company focused on the inflammasome is IFM Therapeutics. In 2019, the major pharmaceutical company Novartis acquired one clinical and two preclinical programs targeting the NLRP3 inflammasome from IFM Therapeutics. One of the preclinical programs was a brain-penetrant molecule which is being developed for targetting neurodegenerative conditions like Alzheimer’s and Parkinson’s (Source). A third inflammation focused biotech company called NodThera has very recently identified its lead NLRP3 targetting drug candidate, called NT-0167 which the company is now developing for clinical evaluation (Click here to read more about this). And late last year, Genentech, a subsidiary of Swiss pharma giant Roche, bought up Jecure Therapeutics – a biotech firm with a portfolio of preclinical NLRP3 inhibitors aimed at various inflammatory conditions (it is fair to say that this biotech was primarily looking at liver inflammation, but perhaps a neuro-focused pharma like Roche will also explore potential neurodegenerative applications). And NLRP3 inhibitors are not the only inflammatory approaches being explored for Parkinson’s. |
In addition to these inflammasome targeting approaches, there are other anti-inflammatory treatments being clinically tested.
In 2020, one such anti-inflammatory clinical trial will be initiated here in the UK. Researchers at Cambridge University will be testing the immunosuppressive medication, Azathioprine, in people with Parkinson’s. We will learn more about the details of the “AZA-PD” trial early in 2020 (Click here to read more about this trial).
(The Cure Parkinson’s Trust is a supporter of the AZA-PD study)
One inflammation-related biotech company that we are watching in 2020 is Inmune Bio.
This biotech firm is developing a drug called XPro1595, which targets soluble TNF. Tumor Necrosis Factor (TNF) is a potent immune signaling molecule (a cytokine) and it is intimately involved in inflammation. But Xpro1595 is different to current clinically approved TNF inhibitors, as it only neutralises soluble TNF, while not affecting trans-membrane TNF (this is an important difference). Inmune Bio has conducted a Phase I clinical study of XPro1595 in individuals with moderate Alzheimer’s, which was scheduled to finish in December 2019 (Click here to read more about this trial). If this treatment is well tolerated, it would be interesting to see this drug tested in Parkinson’s.
In 2020, I am also hoping to hear news regarding sargramostim.
In 2017, researchers in Nebraska completed a clinical trial assessing this immunomodulator drug in Parkinson’s, and they reported interesting results (Click here to read a SoPD post about this). It was suggested by the investigators conducting that study, however, that a reformulation of the drug was required as many of the participants developed antibodies to the drug (which could potentially render the therapy useless by blocking its action). In 2020, it would be nice to learn how the reformulation efforts are going.
Phew, you still with me?
You could get lost in a long post like this. Seriously.
Remember to pace yourself. We’re not even half way yet.
We continue:
Now, if one of these ‘direct’ or ‘indirect’ approaches is able to slow or halt Parkinson’s, the next requirement in a ‘curative therapy’ for Parkinson’s is:
2. A neuroprotective agent
Once a drug or a treatment has been determined to slow down the progression of Parkinson’s, it will be necessary to protect the remaining cells and provide a nurturing environment for the third part of the ‘cure’ (Cell replacement therapy – more on that below).
This is where the second component of any ‘cure’ for Parkinson’s – a neuroprotective agent – is required.
Neuroprotection is the area of research that has had the most attention over the years. Drug companies have employed vast resources in this area in the hope of discovering a treatment which will work across conditions (think Alzheimer’s, Parkinson’s, Huntington’s, etc), and thus provide them with tremendous profits. Unfortunately, conditions of the brain have proven to be a lot more complicated than first perceived and cross-condition therapies seem unlikely as we move towards personalisation.
But there has been the hint of a potential neuroprotective effect in one class of drugs for Parkinson’s: GLP-1R agonists.
Neuroprotective approach: GLP-1R agonists
Exenatide is a Glucagon like peptide-1 receptor (or GLP-1R) agonist. This is a class of drug that has traditionally been used for treating diabetes, but has recently been repurposed for Parkinson’s.
After multiple studies suggested neuroprotective properties in models of Parkinson’s, a clinical trial program was intiated, and in 2017, a Phase II Exenatide trial reported the stablisation of Parkinson’s motor features over the course of the 48 week trial (Click here and here to read previous SoPD posts about this).
In the ‘neuroprotection’ section, top of the list of news that we will be looking for in 2020 is the initition of a Phase III clinical trial for Bydureon/Exenatide.
(The Cure Parkinson’s Trust is a supporter of the Exenatide III study)
Given the Phase II results, there are high expectations for the Phase III trial which need to be carefully managed.
While it will be good to see this trial start, it is important to remember that not everyone in the Phase II trial treated with exenatide responded to the drug – that is to say, not everyone taking the treatment experienced benefits. Thus, an important part of this new trial will be trying to identify characteristics of those who do respond compared to those who do not – which could aid in future administration of the drug (if it is eventually approved).
The Phase III trial will involved 200 participants being treated for 2 years and is scheduled to complete in 2024 (Click here to read more about this trial and Click here to see the trial webpage).
There is also a clinical trial evaluating exenatide in people with recently diagnosed Parkinson’s being initiated in Sweden. It is a Phase II study that will recruit 60 participants and follow them for 18 months (Click here to read more about this study).
The University of Florida also has a small clinical trial evaluating exenatide in people with Parkinson’s. This study is scheduled to finish in 2021 (Click here to read more about this study).
In addition to Exenatide, there are additional ongoing clinical trials for other GLP-1R agonists, for example the Lixisenatide trial in France (Click here and here for more information on this study), and the Liraglutide trial in California (Click here and here to read more about this study). Neither of these two ongoing GLP-1R agonist studies will be completing in 2020.
(The Cure Parkinson’s Trust is a supporter of both the Lixisenatide and Liraglutide studies)
In addition, there is a clinical trial of a new formulation of Exenatide called Semaglutide in Parkinson’s. This study is being conducted in Norway by the Danish biotech firm Novo Nordisk.
This Phase II study will involve 120 participants, who will take either Semaglutide (or placebo) for 24 months in a double blind fashion, before a further 24 months of open label assessments. The study is scheduled to finish in 2024 (Click here to read more about this study).
In 2019, a new GLP-1R agonist called NLY01 entered the clinical trial process. This drug is being developed by Neuraly Inc.
 A Phase I in healthy volunteers was conducted and completed by Neuraly in 2019 (Click here to read more about that study), and the company has now shifted to testing NLY01 in 200+ people with Parkinson’s in a large Phase II trial. The participants will be evaluated over 36 weeks of treatment, and the study is scheduled to finished in 2021 (Click here to read more about this).
A Phase I in healthy volunteers was conducted and completed by Neuraly in 2019 (Click here to read more about that study), and the company has now shifted to testing NLY01 in 200+ people with Parkinson’s in a large Phase II trial. The participants will be evaluated over 36 weeks of treatment, and the study is scheduled to finished in 2021 (Click here to read more about this).
Another new entrant to the GLP-1R agonist group is Peptron.
They too have conducted Phase I safety testing of their GLP-1R agonist (called PT320 – click here to read more about that trial), and hopefully in 2020 we will hear news about their Phase II/III plans.
Another form of ‘neuroprotective’ treatment that has been tested in Parkinson’s for a long time is neurotrophic factors.
Neuroprotective approach: Neurotrophic factors
Neurotrophic factors are supportive/nurturing proteins that the brain produces naturally. They help to keep cells alive by activating and stimulating specific biological pathways. There are different types of neurotrophic factors which affect different types of neurons in different ways (one of my pet peeves is repetition of a word in a single sentence!).
One neurotrophic factor that has a particularly robust effect on dopamine neurons (the type of neurons most severely affected by Parkinson’s) is GDNF (or Glial cell-derived neurotrophic factor).
In 2019, we saw the publication of the Bristol Phase II GDNF clinical trial results. GDNF is a neurotrophic factor that has had a long roller coaster-like history with Parkinson’s. The results of the Phase II trial indicate that there was no significant improvement in those individuals being treated with GDNF compared to those on the control/placebo treatment (as measured by clinical rating scales – click here to read a SoPD post about these results).
(The Cure Parkinson’s Trust was a supporter of the Bristol GDNF study)
Despite the set back with this GDNF trial, there is another GDNF trial that is investigating a gene therapy form of GDNF. Brain Neurotherapy Bio published the results of a Phase I clinical trial assessing their AAV-based GDNF gene therapy treatment in people with Parkinson’s (Click here to read a SoPD post on this topic).
The company is conducting a follow up open-label safety study of their approach which is starting in 2020 (Click here to read more about this trial). This trial will provide further case for support to conduct a much larger clinical trial.
|
OTHER DEVELOPMENTS TO WATCH FOR IN 2020 REGARDING GDNF: Another company working on GDNF is called Genecode. This biotech is developing orally delivered “small molecules that mimic GDNF and trigger neurotrophic signalling in dopaminergic neurons” (Source). GDNF can not be administered orally as it does not cross the blood brain barrier, but Genecode has recently published data suggesting that their orally administered molecule, called BT13, can access the brain and acts in a similar manner to GDNF (Click here and here to read more about this). |
Another neurotrophic factor that is currently in clinical trial is cerebral dopamine neurotrophic factor (CDNF). This protein is different to GDNF, but has also been shown to have beneficial effects on dopamine neurons (Click here to read a good review on this topic).
This study has been conducted by the biotech firm, Herantis.
The Phase I/II study was evaluating the safety and tolerability of CDNF in people with Parkinson’s. Similar to the Bristol Phase II GDNF study (mentioned above), the drug was injected directly into the brain using an implanted canular system. One-third of the participants received monthly infusions of placebo and two-thirds of the participants received monthly infusions of either mid- or high-doses of CDNF for 6 months (Click here to read more about this trial). Herantis has just announced the completion of this trial (Click here to read more about this).
An extension of that study is now being conducted, with all of the participants receiving monthly infusions of either mid- or high-dose of CDNF for a further 6 months (Click here to read more about that follow-up study). This extension is scheduled to finish in September 2020.
Some of the safety/tolerability data from the first 6 months of the trial were presented the Alzheimer’s/Parkinson’s 2019 meeting in March 2019, and they suggested that the treatment was safe and well tolerated.
In addition to these results, we will also hopefully hear more news from Herantis about their efforts to develop a orally administered form of CDNF in 2020 (Click here to read more about this).
Neuroprotective approach: Mitochondria
Mitochondria are the power stations of each cell, as they provide the cell with the energy that the cell needs to function. They help to keep the lights on. Without them, the party is over and the cell dies.

The activity of mitochondria in many cases of Parkinson’s has been found to be reduced, thus treatments that boost mitochondial activity and make the cell healthier have been proposed as potential neuroprotective approaches for Parkinson’s – making the mitochondria (and the cell) stronger and healthier.
And we have numerous clinical trials testing this idea.
In February 2019, we saw the initiation of a new clinical trial evaluating UDCA (aka Ursodeoxycholic acid or ursodiol). This is a medication for the treatment of gallstones, and it is being repurposed for Parkinson’s. Precinical data suggests the treatment has mitochondrial benefits in models of Parkinson’s, which led to the development of the “UP” study (“UDCA in Parkinson’s” study – Click here to read a SoPD post on the topic).
(The Cure Parkinson’s Trust is a supporter of the UP study)
There is a second clinical trial of UDCA which has been conducted at the University of Minnesota.
This Phase I open label study was designed to assess the safety/tolerability of increasing doses of UDCA, but is not expected to report results until 2022 (Click here to read more about this).
Another mitochondrial-focused clinical trial (the “REPAIR-PD” study) that was initiated in 2019 involves a drug called CNM-Au8, which is being developed by a company called Clene Nanomedicine.
This gold-derived (seriously) treatment acts as a potent anti-oxidant, but it also boosts the energy production in mitochondria (Click here to read an SoPD post about this research). The REPAIR-PD study is scheduled to finish in the mid 2020 (Click here to read more about this trial).
Another mitochondrial-focused clinical trial initiated in 2019 was a drug-repurposing study involving the prostatic hyperplasia and hypertension drug, Terazosin.
Researchers published data suggesting that terazosin could rescue models of Parkinson’s by boosting energy production in mitochondria. A 12 week Phase II clinical trial for Terazosin in Parkinson’s was set up to assess safety of the drug in peope with Parkinson’s, and it is scheduled to finish in 2020 (Click here to read about the clinical trial and click here to read an SoPD post on this topic).
One mitochondrial-targeting clinical trial I am hoping to see the results of in 2020 is EPI-589.
(The Cure Parkinson’s Trust was a supporter of the EPI-589 study)
This drug is being developed by BioElectron (formerly Edison Pharmaceuticals), and it helps to boost mitochondrial function. The company have conducted a Phase II open label, safety trial for the evaluation of EPI-589 in people with early onset genetic forms of Parkinson’s and also idiopathic Parkinson’s (Click here to learn more about this trial).
And this trial is interesting given the announcement in late 2018 of positive results for an open label Phase II clinical trial of EPI-589 in motor neurone disease/ALS. That study was assessing safety, tolerability, and disease biomarker effect, and the results “provide a strong rationale for the continued development of EPI-589” in ALS (Click here and here to read more about this and click here for the details of the study).
Another compound that has exhibited interesting results in ALS is CuATSM which is being developed by Collaborative Medicinal Development Pty (Click here to read more about the ALS result).
CuATSM is also a highly effective scavenger of a chemical in our bodies called ONOO, which can be very toxic. In addition, there is evidence that the drug also blocks the aggregation of alpha synuclein and has beneficial effects in models of Parkinson’s (Click here to read an example).
In 2019, we learnt the results of a small Phase I clinical trial of CuATSM in Parkinson’s. The study found that 24 weeks of treatment with the drug was well tolerated, and the participants experienced some improvements in their symptoms (Click here to read more about this). This was an open label study, and we are still waiting to see the results published. But it would be positive to see a larger, double blind, placebo controlled study started in 2020.
A clinical trial of Nicotinamide Riboside (a form of Vitamin B3) has started in Norway – it is called the ‘NOPARK’ Study. Nicotinamide Riboside is an important component in energy production and mitochondrial function – we have previously discussed the biology of Nicotinamide Riboside (Click here to read that SoPD post).
 This study is a randomised, double-blind trial involving 200 participants with newly diagnosed Parkinson’s, who will be randomly assigned in an 1:1 ratio to either nicotinamide riboside or placebo treatment for 52 weeks. The study is scheduled to finish in 2021 (click here to read more about this study).
This study is a randomised, double-blind trial involving 200 participants with newly diagnosed Parkinson’s, who will be randomly assigned in an 1:1 ratio to either nicotinamide riboside or placebo treatment for 52 weeks. The study is scheduled to finish in 2021 (click here to read more about this study).
|
OTHER DEVELOPMENTS TO WATCH FOR IN 2020 REGARDING MITOCHONDRIA: Yet another company that recently announced a positive result in ALS is Amylyx. They are developing a drug called AMX0035, which is a combination of sodium phenylbutyrate and tauroursodeoxycholic acid (TUDCA). Amylyx has been testing this drug in ALS and recently announced a significantly poisitve result (Click here to read more about this). The company is keen to evaluate this drug in other neurodegenerative conditions, and it would be interesting to see it tested in Parkinson’s. |
Neuroprotective approach: Statins
In 2020, we are scheduled to get some news regarding the use of statins in Parkinson’s.
Statins are a class of cholesterol lowering drugs, which have demonstrated neuroprotective properties in preclinical models of Parkinson’s (Click here to read a SoPD post on this topic and click here to read a review on this topic).
A clinical trial in Taiwan investigating the statin Lovastatin as a neuroprotective treatment for early stage Parkinson’s was scheduled to finish in late 2019 (Click here to read more about this study), so we may see those results in 2020.
In addition, the much larger PD-STAT study which evaulating the statin Simvastastin in 230+ people with Parkinson’s is being conducted here in the UK (Click here to learn more about this study) is scheduled to finish in late 2020.
(The Cure Parkinson’s Trust is a supporter of the Simvastatin study)
Neuroprotective approach: Iron chelation
Iron accumulation in certain regions of the brain is a common feature in Parkinson’s, and as a result researchers have tested different methods of reducing excess iron levels in models of Parkinson’s as a method of neuroprotection (Click here to read a review on this topic).
 The positive preclinical results have led to clinical trials in people with Parkinson’s evaluating iron chelators – these are a class of drugs that bind to iron and remove it. An example of an iron chelator is a drug called Deferiprone, and there have recently been two large Phase II clinical trials assessing this approach in Parkinson’s.
The positive preclinical results have led to clinical trials in people with Parkinson’s evaluating iron chelators – these are a class of drugs that bind to iron and remove it. An example of an iron chelator is a drug called Deferiprone, and there have recently been two large Phase II clinical trials assessing this approach in Parkinson’s.
The first of these Deferiprone studies was scheduled to finish in late 2019. This study (called the SKY study) recruited 140 people with early Parkinson’s and treated them twice-daily for 9 months with Deferiprone (one of 4 different doses) or a placebo drug (Click here to read more about the details of this study). We will hopefully learn the results of this trial in 2020. This international trial is being conducted by ApoPharma.
The second Deferiprone study is scheduled to finish in 2020 (Source). This trial is called the FAIRPARK II study.
This is a larger trial than the SKY study, with 330+ participants who are being treated with either Deferiprone or a placebo treatment for 9 months (Click here to read more about this).
(The Cure Parkinson’s Trust is a supporter of the FAIRPARK II study)
In 2018, Alterity Therapeutics (formerly PRANA Biotechnology) initiated a clinical trial of their drug PBT434 in healthy individuals.
This was a Phase I study evaluating the safety, tolerability and pharmacokinetics of this treatment, after single and multiple oral dose administration (Click here to read more about that study and click here for a previous SoPD post on this topic).
Neuroprotective approach: Plasma infusions
Recently there has been a series of studies suggesting that plasma – the portion of blood that doesn’t contain cells – from young organisms may contain beneficial properties for older organisms. Not quite Count Dracula, but there has been a lot of research exploring this idea (Click here to read a SoPD post on this topic). There have also been a couple of clinical trials investigating this as an approach for Parkinson’s.
At the end of 2019, the Stanford Parkinson’s Disease Plasma Study (SPDP), which is being conducted at the Stanford Movement Disorders Center, was scheduled to finish.
This was a Phase I open label clinical study testing whether young plasma infusions could be safely used on 15 people with Parkinson’s. Participants recieved 4 twice-per-week infusions of 1 unit of young plasma (from males ages between 18-25) and were then monitored over 8 weeks (Click here to read more about this study). We will hopefully hear the results of this study in 2020.
In addtion, in late 2020, a clinical trial of a product derived from young blood plasma (called GRF6021) is scheduled to finish. This trial is being conducted by a biotech firm named Alkahest. At the end of 2018, the company announced that they have dosed the first participant in a Phase II clinical trial of their product GRF6021 in people with Parkinson’s and cognitive impairments (Click here for the press release). GRF6021 is a ‘plasma fraction’ (evidently made up of approximately 400 proteins derived from young blood – source).
At the end of 2018, the company announced that they have dosed the first participant in a Phase II clinical trial of their product GRF6021 in people with Parkinson’s and cognitive impairments (Click here for the press release). GRF6021 is a ‘plasma fraction’ (evidently made up of approximately 400 proteins derived from young blood – source).
The study is a randomised, double-blind, placebo-controlled Phase II study in 90 people with Parkinson’s and cognitive impairment. It will be assessing the safety and tolerability of GRF6021 over a period of 7 months. The treatment (or placebo) will be administered by intravenous infusion for 5 consecutive days at Week 1 and Week 13 of the study (Click here to read more about the details of this clinical study). This trial is scheduled to finish in November 2020.
Neuroprotective approach: Fecal transplantion
Not to be confused with fetal cell transplantation (which will be discuss below in component #3), fecal transplantation involves attempting to introduce bacteria from the gastrointestinal system of “healthy” individuals into the gastrointestinal system of people with Parkinson’s.
The bacteria of the gut has recently been the focus of intense Parkinson’s research (Click here, here and here to read SoPD posts on this topic). And this fascination with the bacteria of the gut has led to clinical trials exploring the safety of fecal transplantation in people with Parkinson’s.
Two of these trials are scheduled to finish in 2020.
The first of them is being conducted in Israel and involves 100 participants who have been evaluated for 6 months in a open label fashion (Click here to read more about this trial).
And the second fecal transplant study is being conducted in Houston (Texas), and is a smaller pilot study of 12 participants being evaluated over 12 weeks (Click here to read more about this trial).
Neuroprotective approach: Additionals
The following list of clinical trials is labelled as “Neuroprotective approach: Additionals” as they could not be better grouped.
But they are all neuroprotective approaches focused on Parkinson’s.
A large Phase III clinical trial of the effects of Lingzhi (also known as Ganoderma or reishi in Japan) on disease progression in 288 people with recently diagnosed, untreated Parkinson’s is scheduled to finish in late 2020 (Click here to read more about this study). Limited preclinical research has been published in the west with this extract of an oriental fungus in models of Parkinson’s (for examples, click here and here), but the results of this clinical trial could be something to keep an eye out for in 2020.
A Phase II clinical trial testing Ceftriaxone in 106 people with Parkinson’s with dementia is scheduled to finish in late 2020 (Click here to read more about this trial). Ceftriaxone is a cephalosporin antibiotic, which has been reported to have beneficial effects in models of Parkinson’s (Click here and here for examples and click here to read a review about this drug).
South Korean firm Kainos Medicine completed a Phase I clinical study in 2019 evaluating KM-819 – a small molecule inhibitor for FAF1. FAF1 is a protein involved with cell death, so by inhibiting/blocking it the researchers are investigating whether this could be beneficial in Parkinson’s.
The Phase I study was a randomised, double-blind, placebo-controlled dose-escalation study in healthy volunteers, that found that the drug was safe/tolerable with no drug-related SAEs (Click here to read more about this). Perehaps we will see a Phase II clinical trial initiated in 2020?
In 2019, another Korean pharmaceutical company Dong-A ST Co was scheduled to complete a Phase II clinical trial of DA-9805 in people with Parkinson’s (Click here to read more about this trial).
 DA-9805 is a combination of compounds extracted from three dried plant materials (Moutan cortex, Angelica Dahurica root, and Bupleurum root) in a 1:1:1 mixture, which has demonstrated neuroprotective properties in models of Parkinson’s (Click here to read more about that). In 2020, we will hopefully hear news about the results.
DA-9805 is a combination of compounds extracted from three dried plant materials (Moutan cortex, Angelica Dahurica root, and Bupleurum root) in a 1:1:1 mixture, which has demonstrated neuroprotective properties in models of Parkinson’s (Click here to read more about that). In 2020, we will hopefully hear news about the results.
In 2020, a clinical trial of a drug called ANAVEX2-73 is scheduled to finish. The drug is a Sigma-1 agonist (Click here to read a review on Sigma-1 biology) and it is being developed by the biotech company Anavex.
This is a Phase 2, double-blind, placebo-controlled study evaluating the safety, tolerability, and efficacy of ANAVEX2-73 in 120 people with Parkinson’s with dementia. The participants were treated for 14 weeks (Click here to read more about this trial).
|
OTHER DEVELOPMENTS TO WATCH FOR IN 2020 REGARDING ADDITIONALS: In 2020, we will be hoping to see news from the Parkinson’s UK’s virtual biotech firm called Keapstone Therapeutics. Keapstone was set up in late 2017 to develop neuroprotective therapies for Parkinson’s that are focused on compounds that trigger a cellular defence system (specifically NRF2 activators) that helps protect brain cells from oxidative stress (Click here to read a previous SoPD post about this). We will also be hoping to hear news from Prof Englund’s lab regarding new developments of activators of Nurr 1 (Click here to read a SoPD post on this topic). Prof Englund and colleagues have recently been awarded a Michael J Fox foundation research grant to further develop this line of research (Click here to read more about this). |
Wow!
This is like a test of madness.
Onwards:
Above are just some of the neuroprotective clinical trials that are currently being initiated/conducted. I apologise for any that I have left out, but I am primarily focusing on those starting or completing in 2020. If you know of a clinical trial that deserves a mention – please contact me.
And now, we move on to 2020 clinical trials involving the third component of any ‘curative therapy’ for Parkinson’s:
3. Some form of restorative therapy
Once the condition has been slowed/halted (#1) and a neuroprotective/nurturing environment is in place to protect the remaining cells (#2), a curative treatment for Parkinson’s will require replacing some of the cells that have been lost.
By the time a person is presenting the motor features characteristic of Parkinson’s, and being referred to a neurologist for diagnosis, they have already lost approximately 50% of the dopamine producing neurons in an area of the brain called the midbrain. These cells are critical for normal motor function – without them, movement becomes very inhibited.
And until we have developed methods that can identify Parkinson’s long before the motor features appear (which would require only a disease halting treatment), some form of cell replacement therapy is required to introduce new cells to take up lost function.
Cell transplantation currently represents the most straight forward method of cell replacement therapy.
Traditionally, the cell transplantation procedure for Parkinson’s has involved multiple injections of developing dopamine neurons being made into an area of the brain called the putamen (which is where much of the dopamine naturally produced in the brain is actually released). These multiple sites allow for the transplanted cells to produce dopamine in the entire extent of the putamen. And ideally, the cells should remain localised to the putamen, so that they are not producing dopamine in areas of the brain where it is not desired (possibly leading to side effects).

Targeting transplants into the putamen. Source: Intechopen
Postmortem analysis – of the brains of individuals who have previously received transplants of dopamine neurons and then subsequently died from natural causes – has revealed that the transplanted cells can survive the surgical procedure and integrate into the host brain. In the image below, you can see rich brown areas of the putamen in panel A. These brown areas are the dopamine producing cells (stained in brown). A magnified image of individual dopamine producing neurons can be seen in panel B:

Transplanted dopamine neurons. Source: Sciencedirect
The transplanted cells take several years to develop into mature neurons after the transplantation surgery. This means that the actually benefits of the transplantation technique will not be apparent for some time (2-3 years on average). Once mature, however, it has also been demonstrated (using brain imaging techniques) that these transplanted cells can produce dopamine. As you can see in the images below, there is less dopamine being processed (indicated in red) in the putamen of the Parkinsonian brain on the left than the brain on the right (several years after bi-lateral – both sides of the brain – transplants):

Brain imaging of dopamine processing before and after transplantation. Source: NIH
The old fashioned approach to cell transplantation involved dissecting out the region of the developing dopamine neurons from multiple donor embryos (these are referred to as fetal transplants – different to the fecal transplants discussed further above). The tissue was broken into small pieces that could be passed through a tiny syringe, and this was injected into the brain of a person with Parkinson’s.
The people receiving this sort of transplant would require ‘immunosuppression treatment’ for long periods of time after the surgery. This additional treatment involves taking drugs that suppress the immune system’s ability to defend the body from foreign agents. This step is necessary, however, in order to stop the body’s immune system from attacking the transplanted cells (which would not be considered ‘self’ by the immune system), allowing those cells to have time to mature, integrate into the brain and produce dopamine.
|
SMALL SIDE NOTE HERE: It is very important for all readers of this post to appreciate that cell transplantation for Parkinson’s is still extremely experimental. Anyone declaring otherwise (or trying selling a procedure based on this approach) should not be trusted. There are no cell therapy approaches for Parkinson’s that have been approved by any medical regulators. While I appreciate the desperate desire of the Parkinson’s community to treat this condition ‘by any means possible’, bad or poor outcomes for this technology could have serious consequences for the individuals receiving the procedure and negative ramifications for all future research in the stem cell transplantation area. |
There is an ongoing European clinical trial called Transeuro which is using the fetal cell approach for Parkinson’s.
(The Cure Parkinson’s Trust is a supporter of the TRANSEURO study)
This trial is an open label study, involving 13 subjects. This study is not scheduled to finish until 2021 (Click here to read more about this).
There is also a South Korean clinical trial being conducted at Bundang CHA Hospital, assessing fetal dopamine neurons in 15 participants and evaluating them over 5 years. That study is not scheduled to finish until 2022 (Click here to read more about this).
In addition to these two fetal cell transplantation studies, there are currently 4 ongoing stem cell-based clinical trials for Parkinson’s.
The first of those clinical trials is being conducted in Melbourne (Australia), by an American company called International Stem Cell Corporation (ISCO).

This study is taking the new approach to cell transplantation, and the company is using a different type of stem cell to produce dopamine neurons for transplantion in the Parkinsonian brain (Click here and here to read previous posts about this).
Specifically, the researchers will be transplanting human parthenogenetic stem cells-derived neural stem cells (hpNSC). These hpNSCs come from an unfertilized egg – that is to say, no sperm cell is involved. The female egg cell is chemically encouraged to start dividing and then it becoming a collection of cells that is called a blastocyst, which ultimately go on to contain embryonic stem cell-like cells.

This process is called ‘Parthenogenesis’, and it’s not actually as crazy as it sounds as it occurs naturally in some plants and animals (Click here to read more about this). Proponents of the parthenogenic approach suggest that this is a more ethical way of generating embryonic cells as it does not result in the destruction of a viable organism. The stem cells derived from this process can be grown into neuronal cells before being transplanted into brain.
The ISCO trial involves 12 participants who have been evaluated over 12 months in this evaluation of safety and tolerability. The study is scheduled to finish in 2020 (Click here to read more about this trial).
In 2018, a second stem cell-based cell transplantation clinical trial was initiated by a research group from China led by Professor Qi Zhou, a stem-cell specialist at the Chinese Academy of Sciences Institute of Zoology.
This study is taking place at The First Affiliated Hospital of Zhengzhou University in Henan province, and involves 10 participants being injected with neuronal-precursor cells derived from embryonic stem cells (Click here to read more about this trial). The study is scheduled to finish in late 2020 (Click here to read a previous SoPD post on this topic).
A second Chinese cell transplantation trial is also registered, and being conducted at The First People’s Hospital of Yunnan Province. This study of 10 participants is also a safety trial and is scheduled to finish in 2021 (Click here to read more about this study).
And the fourth on-going stem cell-based cell transplantation clinical trial is the ‘induced pluripotent stem’ (or IPS) cell study being conducted in Kyoto, Japan (Click here to read a SoPD post on this topic).
This ongoing cell transplantation trial is being conducted by researchers at the Center for iPS Cell Research and Application (or CiRA).
 In 2017, the researchers behind this trial published a report evaluating their IPS cells in primate models of Parkinson’s (Click here to read more about that research report).
In 2017, the researchers behind this trial published a report evaluating their IPS cells in primate models of Parkinson’s (Click here to read more about that research report).
Information provided by Kyoto University (Click here for the study website) and the Japanese media has indicated that this Phase I/II clinical trial aims to investigate the safety and efficacy of transplanting human IPS cell-derived dopaminergic progenitors into the brains of people with Parkinson’s (what stage of PD has not been disclosed). The study will involve just 7 participants, who are all Japanese individuals with Parkinson’s. The participants in the study will be followed and assessed for 2 years post transplantation
According to the CIRA 2018 annual report, the researchers began enrolling patients on 1st August and the first patient was transplanted in October (2018 – Click here to read the annual review). The investigators stated that the gentleman who was transplanted will be monitored for 2 years…which is October 2020.
|
OTHER DEVELOPMENTS TO WATCH FOR IN 2020 REGARDING REGENERATIVE THERAPIES: In the summer of 2019, the pharmaceutical company Bayer acquired a biotech company called BlueRock Therapeutics (Click here to read a SoPD post on this). BlueRock is a stem-cell therapy company focused on induced pluripotent stem cell (iPSC)-derived therapeutics for cardiovascular disease and neurodegenerative disorders, particularly Parkinson’s (co-founders Lorenz Studer and Viviane Tabar are world renowned experts in the field of cell transplantation for Parkinson’s). The company is setting up a trial (to be called STEM-PD), which will be an open label Phase I/IIa safety and tolerability study involving 10 individuals with moderate to severe Parkinson’s (5-10 years since diagnosis). In 2020, we are hoping to hear news of the initiation of this trial. In addition to BlueRock/Bayer, there are additional entrants to the area of cell transplantation for Parkinson’s, including Aspen Neuroscience. The company recently launched with seed funding to advance their personalized cell transplantation therapy approach for Parkinson’s (Click here to read the press release). In 2020, we will be hoping to hear news regarding the development of the clinical program of this biotech firm’s first product ANPD001 – an autologous neuron replacement therapy in Parkinson’s. And I would like to learn more about their second product (ANPD002) which is “a gene-edited autologous neuron replacement therapy for familial forms of Parkinson’s” (Source). On top of these biotech companies, there are additional firms quietly preparing to enter this space. In 2020, I will be curious to see if companies like Fujifilm/Cellular Dynamics or Novo Nordisk step up to the plate and start a clinical trial program focused on cell transplantation for Parkinson’s. |
Ok, that’s it.
I think we are done.
There are other bits and pieces that I am not at liberty to discuss, but all of the rest of the information above should give you a frame work for what is scheduled for the next 12 months with regards to experimental disease modifying therpies for Parkinson’s.
One complaint regarding this post will be that all of these trials are focused on drugs/gene therapies/etc. It is a fair comment, but broadening the scope of this post would be the death of me I suspect. For those interested in exercise, there are a lot of clinical trials that have just registered or recruiting (78 at the time of writing) which could be explored (Click here to read more about these).
Before we finish (yes, there is still more!), I thought I would mention a couple of other events that are occurring in 2020 that readers may be interested in:
One of my favourite meetings of the year is the Grand Challenges meeting in Grand Rapids, Michigan.
The theme this year is ‘When and how does Parkinson’s start‘.
This meeting “will focus on the origins and development of Parkinson’s, from potential triggers through the early symptoms and stages of the disease“.
The beauty of this meeting is that it is a two for one deal – while there are scientific research lectures on one side of the conference center, at the same time there is the Rallying to the Challenge meeting being held on the other side. This additional meeting is designed for and run by people living with Parkinson’s. ‘Rallying’ (as it is affectionately known) has one simple aim: to involve people living with Parkinson’s in research. It is an excellent opportunity to come and learn about all the different ways of getting involved in PD research, without necessarily getting involved.
These are two meetings that I can thoroughly recommend to readers who have never attended such an event.
(FULL DISCLOSURE: The Rallying to the Challenge meeting is a Cure Parkinson’s Trust supported event and the author of this blog is an employee of The Cure Parkinson’s Trust – so he may be a little biased)
Here in the UK, I am looking forward to taking part in the 3rd Dundee Edinburgh Parkinson’s Research Initiative Symposium on the 20th March.
And am also to going to be speaking at the Parkinson’s UK supported 9th Annual East Midlands Parkinson’s Research Forum 2020.
The meeting will be held on the 24th October at the Link Hotel Loughborough. It is always a good meeting – focused on explaining the research in plain English – and this year has a fastastic line up of speakers (I’m just second or third fiddle). Will add more information here when it becomes available.
Summary
I am regularly asked by members of the Parkinson’s community to provide some kind of an overview of everything that is going on research wise for Parkinson’s. I hope that this meandering shambles of just a portion of the clinical research on disease modification has illustrated what an impossible task that would be. In addition, what is not apparent from a static view like this post is that the landscape is always changing – so quickly in fact that I know within a few days of publishing this post, there will be new announcements further changing that landscape. Perhaps “landscape” is the wrong word to use.
I would be remise if I did not make one last statement regarding the need to check expectations. While this mammoth list of clinical trials bodes well for the future of Parkinson’s treatments, there are no guarantees that any of them are actually going to work. In addition, we must remember that a clinical trial is supposed to be an unbiased evaluation of a treatment. If we enter a clinical trial with any expectation (as a participant or observer), we are by definition biased. We should approach all of these trials with no expectations, and evaluate the outcomes not based on success or failure, but on what we have learnt (and yes, I appreciate that is easy to say for one who is not affected by the condition).
The amount of research being conducted is a very positive sign, however, and it suggests that 2020 will be an extremely productive year of Parkinson’s research. That said, there are certain things that could be improved. In the next post, we will discuss what I personally am hoping to see more of in Parkinson’s research in 2020 (and I promise you it will be a lot shorter than this current post).
But now it’s time to give the fingers (and brain) a wee rest.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, some of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
Further, the author of this post is an employee of the Cure Parkinson’s Trust. The Trust has not asked for this post to be written, and there has been no effort to highlight the work of the Trust over others (perceptions of any bias should be directed to the author). This post has been written by the author solely for the purpose of sharing what the author considers interesting information.
The banner for today’s post was sourced from Weknowyourdreams



























































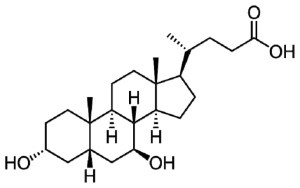





















Excellent blog post, Simon. I would argue that the Abelson tyrosine kinase inhibitors can be both neuroprotective and disease halting ( if they work). They activate autophagy pathways that break down the aggregates. That theoretically ensures cell survival as there there’s no oxidative stress and some of the other bad actors that cause cell death, and also no aggregates means no sense to sell transmission of aggregates. The major downside to these treatments is that they will have to be chronic.
Also, Voyager and Abbvie have a signed a deal to develop a gene therapy to code for an antibody against intracellular synuclein, as opposed to extracellular antibodies that current clinical approaches are targeting to prevent cell to cell transfer at the synapse. Again this is not for 2020 and it’s still pre clinical.
Much progress is expected in the gene therapy sphere too in 2020. Axovant Will be announcing the progress from their trial in H1. A lot of excitement in Voyager AADC considering the RMAT designation
LikeLike
Hi Deepak,
Thanks for the interesting comment. There are quite a few molecules and classes of drugs that can sit across different domains. The current arrangement was simply the easiest way I could find to sort them into some kind of logical order. Some experimental therapies (such as some of the herbal extracts or the plasma infusions) are extremely difficult to place in any particular grouping.
The Axovant & voyager work is exciting, but I left them out here as they are primarily symptomatic plays as opposed to the potentially disease modifying efforts (like the Brain Neurotherapy Bio or Prevail trials).
Kind regards,
Simon
LikeLike
Also are you aware of any synuclein knock down strategies like an ASO or an RNA interference molecule for sporadic PD? Prevail has one in pre clinical for genetic PD. With not knowing what role the protein plays in the norm lal brain I would expect it to be some sort of partial KD
LikeLike
Simon, Your hard work is much appreciated, thank you. Although the trials are inevitably of a jam tomorrow nature I am reassured that my DIY “Amazon regime” might be on the right track of slowiing progression ( of iPD in a 74 yrs male). The ingredients I buy online are all endogenous and target pathways you outline above.
mTOR and autophagy; lipoic acid, acyl carnitine, vit D3, acting via AMPK
nrf2 and antioxidants: vit D3, lipoic acid, melatonin
Mitochondria: co-Q10, acyl carnitine,
Anti-inflammatory: vit D3
Also:
Resolvins: omega fatty acids+low dose aspirin
VitK2Mk4Mk7 ; directs extra calcium uptake caused by D3 to bones
magnesium
choline
multivitamin
I am also to date naive of rasagiline etc
Placebo effect probably great, but if it works, fine.
Peter
LikeLike
Fujifilm Cellular Dynamics anticipate a trial commencement later this year. Look around at the 7 minute mark in this video. https://youtu.be/VdKIRqkMOtI . Another team looking to enter the clinic soon although I don’t know when is Harvard medical School (McLane Hospital), although I’m not sure what corporate partnerships they have to advance their therapy to the clinic. https://youtu.be/PqLL9sIidZg
LikeLike
Wow! Deepak – the Fujifilm video is a great find. IND in 2020?!? That is interesting. Thanks for sharing.
Prof Schweitzer has published some work in the field of cell therapy for Parkinson’s (for example, https://www.jci.org/articles/view/130767 ), he speaks English, French and Mandarin Chinese, and it will be interesting to watch what happens in Boston with their research. I was not aware that they were contemplating translation to the clinic so soon. Very interesting.
Kind regards,
Simon
LikeLike
Autologous at Harvard? WOW! Aspen appears to have nailed down how to make autologous neurons cost-effective.
https://endpts.com/stem-cell-pioneer-jeanne-loring-tosses-hat-into-quickening-parkinsons-race/
“You would need to have a highly robust, bulletproof, cell replication system,” Scott said.
Loring says she’s developed that. Leaning on her genomics background and a machine learning, she says she’s built technology to standardize the process. She argues that she can save money by manufacturing far fewer cell lines.
LikeLike
A valiant effort , Simon . Well done!.
I was disappointed that nothing mentioned about Parkinsons Plus / Atypical Parkinsons..
LikeLike
Hi Keith,
Thanks. Unfortunately, there is only so much we can cover here. But there is a lot of activity in the Parkinson’s Plus domain: if you haven’t already, have a look at the Cambridge Centre for Parkinson-Plus ( https://ccpp.cam.ac.uk/ ) at Cambridge University which has set up several clinical trials late last year thanks to a generous donation.
I hope all is well.
Simon
LikeLike
What a list, thank you for doing all the filtering. Expectations under control but looking forward to the rest of 2020 and beyond…
LikeLike
Hi Martin,
You are welcome. Glad you liked it.
Kind regards,
Simon
LikeLike
Thanks as always Simon. This is now my fourth “Road Ahead” and it’s nice to see the progress. It never of course will be as fast as I want 🙂
You weren’t kidding about the timeline on ambroxol. My guess is we’ll see a post in the next 72 hours!
LikeLike
Hi Double,
As always you are most welcome.
Like I said in the post, the landscape is always changing. Just look at today, Denali reporting their Phase Ib results (https://apnews.com/Globe%20Newswire/2807132693960b18facfe189d4359313) AND Affirirs putting out a press release saying Phase II is in the works (https://parkinsonsnewstoday.com/2020/01/14/affiris-phase-2-study-potential-parkinsons-vaccine/).
The Ambroxol post will have to wait a few days I’m afraid, bit too busy on this end, but this is a good summary of the results: https://www.cureparkinsons.org.uk/news/ambroxol-phase-ll-results-published
Kind regards,
Simon
LikeLike
Thanks Simon! More to read!
LikeLike
Simon,
Also note that there another company called Anavex Life Sciences (AVXL) that is targeting various Central Nervous System Diseases (AD, PD, Rett, FTD ). They are currently targeting PD Dementia by conducting trials (phase 2) in Spain and US with read out in mid 2020. More details of the Mechanism of Action, Presentations, Team, Pipeline and Current Trials etc. can be found at their website.
https://www.anavex.com/#!/home
LikeLike
Ashamed to say I’ve only just read this blog. Thank you so much, Simon, for the astounding amount of work you’ve put into it. Fingers crossed for 2020.
LikeLike
As a recently diagnosed woman with Parkinson’s- your blog is like a scientific torch on my hurry-up and learn approach to understanding this disease. My expectations are enthusiastically under control.
LikeLike